Kinetic structure of large-conductance Ca2+-activated K+ channels suggests that the gating includes transitions through intermediate or secondary states. A mechanism for flickers
- PMID: 9607935
- PMCID: PMC2217154
- DOI: 10.1085/jgp.111.6.751
Kinetic structure of large-conductance Ca2+-activated K+ channels suggests that the gating includes transitions through intermediate or secondary states. A mechanism for flickers
Abstract
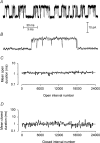
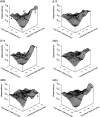
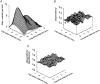
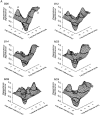
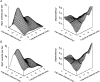
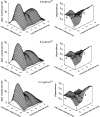
Mechanisms for the Ca2+-dependent gating of single large-conductance Ca2+-activated K+ channels from cultured rat skeletal muscle were developed using two-dimensional analysis of single-channel currents recorded with the patch clamp technique. To extract and display the essential kinetic information, the kinetic structure, from the single channel currents, adjacent open and closed intervals were binned as pairs and plotted as two-dimensional dwell-time distributions, and the excesses and deficits of the interval pairs over that expected for independent pairing were plotted as dependency plots. The basic features of the kinetic structure were generally the same among single large-conductance Ca2+-activated K+ channels, but channel-specific differences were readily apparent, suggesting heterogeneities in the gating. Simple gating schemes drawn from the Monod- Wyman-Changeux (MWC) model for allosteric proteins could approximate the basic features of the Ca2+ dependence of the kinetic structure. However, consistent differences between the observed and predicted dependency plots suggested that additional brief lifetime closed states not included in MWC-type models were involved in the gating. Adding these additional brief closed states to the MWC-type models, either beyond the activation pathway (secondary closed states) or within the activation pathway (intermediate closed states), improved the description of the Ca2+ dependence of the kinetic structure. Secondary closed states are consistent with the closing of secondary gates or channel block. Intermediate closed states are consistent with mechanisms in which the channel activates by passing through a series of intermediate conformations between the more stable open and closed states. It is the added secondary or intermediate closed states that give rise to the majority of the brief closings (flickers) in the gating.
Figures






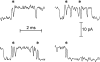





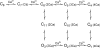

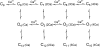
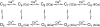





References
-
- Ackers GK, Doyle ML, Myers D, Daugherty MA. Molecular code for cooperativity in hemoglobin. Science. 1992;255:54–63. - PubMed
-
- Adelman JP, Shen E, Kavanaugh MP, Warren RA, Wu Y, Lagrutta A, Bond C, North RA. Calcium-activated potassium channels expressed from cloned complementary DNAs. Neuron. 1992;9:209–216. - PubMed
-
- Atkinson NS, Robertson GA, Ganetzky B. A component of calcium-activated potassium channels encoded by the Drosophila slo locus. Science. 1991;253:551–555. - PubMed
-
- Ball FG, Sansom MSP. Ion-channel gating mechanisms: model identification and parameter estimation from single channel recordings. Proc R Soc Lond B Biol Sci. 1989;236:385–416. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

