Electrophysiological characterization of the rat epithelial Na+ channel (rENaC) expressed in MDCK cells. Effects of Na+ and Ca2+
- PMID: 9607939
- PMCID: PMC2217156
- DOI: 10.1085/jgp.111.6.825
Electrophysiological characterization of the rat epithelial Na+ channel (rENaC) expressed in MDCK cells. Effects of Na+ and Ca2+
Abstract
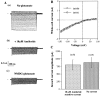
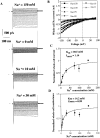
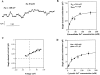
The epithelial Na+ channel (ENaC), composed of three subunits (alpha, beta, and gamma), is expressed in several epithelia and plays a critical role in salt and water balance and in the regulation of blood pressure. Little is known, however, about the electrophysiological properties of this cloned channel when expressed in epithelial cells. Using whole-cell and single channel current recording techniques, we have now characterized the rat alpha beta gamma ENaC (rENaC) stably transfected and expressed in Madin-Darby canine kidney (MDCK) cells. Under whole-cell patch-clamp configuration, the alpha beta gamma rENaC-expressing MDCK cells exhibited greater whole cell Na+ current at -143 mV (-1,466.2 +/- 297.5 pA) than did untransfected cells (-47.6 +/- 10.7 pA). This conductance was completely and reversibly inhibited by 10 microM amiloride, with a Ki of 20 nM at a membrane potential of -103 mV; the amiloride inhibition was slightly voltage dependent. Amiloride-sensitive whole-cell current of MDCK cells expressing alpha beta or alpha gamma subunits alone was -115.2 +/- 41.4 pA and -52.1 +/- 24.5 pA at -143 mV, respectively, similar to the whole-cell Na+ current of untransfected cells. Relaxation analysis of the amiloride-sensitive current after voltage steps suggested that the channels were activated by membrane hyperpolarization. Ion selectivity sequence of the Na+ conductance was Li+ > Na+ >> K+ = N-methyl-D-glucamine+ (NMDG+). Using excised outside-out patches, amiloride-sensitive single channel conductance, likely responsible for the macroscopic Na+ channel current, was found to be approximately 5 and 8 pS when Na+ and Li+ were used as a charge carrier, respectively. K+ conductance through the channel was undetectable. The channel activity, defined as a product of the number of active channel (n) and open probability (Po), was increased by membrane hyperpolarization. Both whole-cell Na+ current and conductance were saturated with increased extracellular Na+ concentrations, which likely resulted from saturation of the single channel conductance. The channel activity (nPo) was significantly decreased when cytosolic Na+ concentration was increased from 0 to 50 mM in inside-out patches. Whole-cell Na+ conductance (with Li+ as a charge carrier) was inhibited by the addition of ionomycin (microM) and Ca2+ (1 mM) to the bath. Dialysis of the cells with a pipette solution containing 1 microM Ca2+ caused a biphasic inhibition, with time constants of 1.7 +/- 0.3 min (n = 3) and 128.4 +/- 33.4 min (n = 3). An increase in cytosolic Ca2+ concentration from <1 nM to 1 microM was accompanied by a decrease in channel activity. Increasing cytosolic Ca2+ to 10 microM exhibited a pronounced inhibitory effect. Single channel conductance, however, was unchanged by increasing free Ca2+ concentrations from <1 nM to 10 microM. Collectively, these results provide the first characterization of rENaC heterologously expressed in a mammalian epithelial cell line, and provide evidence for channel regulation by cytosolic Na+ and Ca2+.
Figures
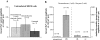









References
-
- Barry PH, Lynch JW. Liquid junction potentials and small cell effects in patch-clamp analysis. J Membr Biol. 1991;121:101–117. - PubMed
-
- Canessa CM, Horisberger J-D, Rossier BC. Epithelial sodium channel related to proteins involved in neurodegeneration. Nature. 1993;361:467–470. - PubMed
-
- Canessa CM, Schild L, Buell G, Thorens B, Gautschi I, Horisberger J-D, Rossier BC. Amiloride-sensitive epithelial Na+channel is made of three homologous subunits. Nature. 1994a;367:463–467. - PubMed
-
- Canessa CM, Mérillat A-M, Rossier BC. Membrane topology of the epithelial sodium channel in intact cells. Am J Physiol. 1994b;267:C1682–C1690. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

