Quality control of synovial fluid crystal identification
- PMID: 9613340
- PMCID: PMC1752532
- DOI: 10.1136/ard.57.2.107
Quality control of synovial fluid crystal identification
Abstract
Objective: To establish a quality assessment programme for the diagnosis of crystal arthropathies by synovial fluid (SF) microscopy.
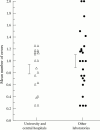
Methods: Three or four cytocentrifuge slides prepared from suitable patient SF specimens were distributed to 25-47 predominantly Finnish clinical laboratories once a year. Sodium urate crystals were included in every survey.
Results: Returns for the years 1989-1996 were reviewed. Laboratories that participated in > four surveys made on an average one error a year (range 0.25-2). The error rate for specimens containing abundant crystals was acceptable but it increased considerably for specimens showing few crystals per microscope field. No laboratory characteristic predictive of successful performance was found.
Conclusion: Errors in quality assessment results for crystal identification were much more frequent than in the fields of, for example, clinical chemistry or microbiology. Despite efforts to provide educational feedback, no improvement was seen during the study period. Because of the dearth of data from other parts of the world it is not known for certain whether this study has merely pinpointed a local problem or if the same trend applies elsewhere.
Figures
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical