B-50/GAP-43-induced formation of filopodia depends on Rho-GTPase
- PMID: 9614174
- PMCID: PMC25350
- DOI: 10.1091/mbc.9.6.1279
B-50/GAP-43-induced formation of filopodia depends on Rho-GTPase
Abstract
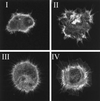
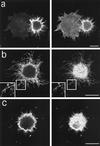
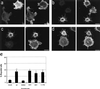
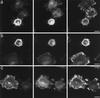
In the present study we show that expression of the neural PKC-substrate B-50 (growth-associated protein [GAP-43]) in Rat-1 fibroblasts induced the formation of filopodial extensions during spreading. This morphological change was accompanied by an enhanced formation of peripheral actin filaments and by accumulation of vinculin immunoreactivity in filopodial focal adhesions, colocalizing with B-50. In time lapse experiments, the B-50-induced filopodial extensions were shown to stay in close contact with the substratum and appeared remarkably stable, resulting in a delayed lamellar spreading of the fibroblasts. The morphogenetic effects of the B-50 protein were entirely dependent on the integrity of the two N-terminal cysteines involved in membrane association (C3C4), but were not significantly affected by mutations of the PKC-phosphorylation site (S41) or deletion of the C terminus (177-226). Cotransfection of B-50 with dominant negative Cdc42 or Rac did not prevent B-50-induced formation of filopodial cells, whereas this process could be completely blocked by cotransfection with dominant negative Rho or Clostridium botulinum C3-transferase. Conversely, constitutively active Rho induced a similar filopodial phenotype as B-50. We therefore propose that the induction of surface extensions by B-50 in spreading Rat-1 fibroblasts depends on Rho-guanosine triphosphatase function.
Figures

References
-
- Aarts LHJ, Van der Linden JAM, Hage WJ, Van Rozen AJ, Oestreicher AB, Gispen WH, Schotman P. N-terminal cysteines essential for Golgi sorting of B-50 (GAP-43) in PC12 cells. Neuroreport. 1995;6:969–972. - PubMed
-
- Aigner L, Arber S, Kapfhammer JP, Laux T, Schneider C, Botteri F, Brenner HR, Caroni P. Overexpression of the neural growth-associated protein GAP-43 induces nerve sprouting in the adult nervous system of transgenic mice. Cell. 1995;83:269–278. - PubMed
-
- Apel ED, Litchfield DW, Clark RH, Krebs EG, Storm DR. Phosphorylation of neuromodulin (GAP-43) by casein kinase II; identification of phosphorylation sites and regulation by calmodulin. J Biol Chem. 1991;16:10544–10551. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

