Virulence and functions of myosin II are inhibited by overexpression of light meromyosin in Entamoeba histolytica
- PMID: 9614192
- PMCID: PMC25380
- DOI: 10.1091/mbc.9.6.1537
Virulence and functions of myosin II are inhibited by overexpression of light meromyosin in Entamoeba histolytica
Abstract
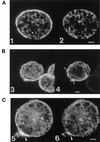
Several changes in cell morphology take place during the capping of surface receptors in Entamoeba histolytica. The amoebae develop the uroid, an appendage formed by membrane invaginations, which accumulates ligand-receptor complexes resulting from the capping process. Membrane shedding is particularly active in the uroid region and leads to the elimination of accumulated ligands. This appendage has been postulated to participate in parasitic defense mechanisms against the host immune response, because it eliminates complement and specific antibodies bound to the amoeba surface. The involvement of myosin II in the capping process of surface receptors has been suggested by experiments showing that drugs that affect myosin II heavy-chain phosphorylation prevent this activity. To understand the role of this mechanoenzyme in surface receptor capping, a myosin II dominant negative strain was constructed. This mutant is the first genetically engineered cytoskeleton-deficient strain of E. histolytica. It was obtained by overexpressing the light meromyosin domain, which is essential for myosin II filament formation. E. histolytica overexpressing light meromyosin domain displayed a myosin II null phenotype characterized by abnormal movement, failure to form the uroid, and failure to undergo the capping process after treatment with concanavalin A. In addition, the amoebic cytotoxic capacities of the transfectants on human colon cells was dramatically reduced, indicating a role for cytoskeleton in parasite pathogenicity.
Figures

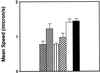
 ) and LMM20
faster (
) and LMM20
faster ( ). The same type of representation was chosen for LMM30:
slower (▥) and LMM30 faster (▨). Finally, the mean speeds for
ExEh20 (□) and wild type (▪) are represented.
). The same type of representation was chosen for LMM30:
slower (▥) and LMM30 faster (▨). Finally, the mean speeds for
ExEh20 (□) and wild type (▪) are represented.


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

