Identification of four classes of brain nicotinic receptors using beta2 mutant mice
- PMID: 9614223
- PMCID: PMC6792706
- DOI: 10.1523/JNEUROSCI.18-12-04461.1998
Identification of four classes of brain nicotinic receptors using beta2 mutant mice
Abstract
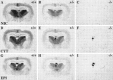
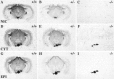
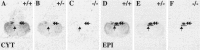
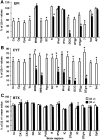
Although the expression patterns of the neuronal nicotinic acetylcholine receptor (nAChR) subunits thus far described are known, the subunit composition of functional receptors in different brain areas is an ongoing question. Mice lacking the beta2 subunit of the nAChR were used for receptor autoradiography studies and patch-clamp recording in thin brain slices. Four distinct types of nAChRs were identified, expanding on an existing classification [Alkondon M, Albuquerque EX (1993) Diversity of nicotinic acetylcholine receptors in rat hippocampal neurons. I. Pharmacological and functional evidence for distinct structural subtypes. J Pharmacol Exp Ther 265:1455-1473.], and tentatively identifying the subunit composition of nAChRs in different brain regions. Type 1 nAChRs bind alpha-bungarotoxin, are not altered in beta2 -/- mice, and contain the alpha7 subunit. Type 2 nAChRs contain the beta2 subunit because they are absent in beta2 -/- mice, bind all nicotinic agonists used with high affinity (excluding alpha-bungarotoxin), have an order of potency for nicotine >> cytisine in electrophysiological experiments, and are likely to be composed of alpha4 beta2 in most brain regions, with other alpha subunits contributing in specific areas. Type 3 nAChRs bind epibatidine with high affinity in equilibrium binding experiments and show that cytisine is as effective as nicotine in electrophysiological experiments; their distribution and persistence in beta2 -/- mice strongly suggest a subunit composition of alpha3 beta4. Type 4 nAChRs bind cytisine and epibatidine with high affinity in equilibrium binding experiments and persist in beta2 -/- mice; cytisine = nicotine in electrophysiological experiments. Type 4 nAChRs also exhibit faster desensitization than type 3 nAChRs at high doses of nicotine. Knock-out animals lacking individual alpha subunits should allow a further dissection of nAChR subclasses.
Figures







Similar articles
-
Relating neuronal nicotinic acetylcholine receptor subtypes defined by subunit composition and channel function.Mol Pharmacol. 2003 Feb;63(2):311-24. doi: 10.1124/mol.63.2.311. Mol Pharmacol. 2003. PMID: 12527802
-
High-affinity epibatidine binding of functional, human alpha7-nicotinic acetylcholine receptors stably and heterologously expressed de novo in human SH-EP1 cells.J Pharmacol Exp Ther. 2005 Apr;313(1):24-35. doi: 10.1124/jpet.104.079004. Epub 2004 Dec 8. J Pharmacol Exp Ther. 2005. PMID: 15590768
-
The influence of nicotinic receptor subunit composition upon agonist, alpha-bungarotoxin and insecticide (imidacloprid) binding affinity.Neuropharmacology. 2000 Feb 14;39(4):671-9. doi: 10.1016/s0028-3908(99)00170-7. Neuropharmacology. 2000. PMID: 10728888
-
Pharmacology of neuronal nicotinic acetylcholine receptor subtypes.Adv Pharmacol. 1997;39:191-220. doi: 10.1016/s1054-3589(08)60072-1. Adv Pharmacol. 1997. PMID: 9160116 Review.
-
Molecular mechanisms underlying behaviors related to nicotine addiction.Cold Spring Harb Perspect Med. 2013 Jan 1;3(1):a012112. doi: 10.1101/cshperspect.a012112. Cold Spring Harb Perspect Med. 2013. PMID: 23143843 Free PMC article. Review.
Cited by
-
Presynaptic α4β2 nicotinic acetylcholine receptors increase glutamate release and serotonin neuron excitability in the dorsal raphe nucleus.J Neurosci. 2012 Oct 24;32(43):15148-57. doi: 10.1523/JNEUROSCI.0941-12.2012. J Neurosci. 2012. PMID: 23100436 Free PMC article.
-
Potentiation of a neuronal nicotinic receptor via pseudo-agonist site.Cell Mol Life Sci. 2019 Mar;76(6):1151-1167. doi: 10.1007/s00018-018-2993-7. Epub 2019 Jan 1. Cell Mol Life Sci. 2019. PMID: 30600358 Free PMC article.
-
The retrotrapezoid nucleus and the neuromodulation of breathing.J Neurophysiol. 2021 Mar 1;125(3):699-719. doi: 10.1152/jn.00497.2020. Epub 2020 Dec 2. J Neurophysiol. 2021. PMID: 33427575 Free PMC article. Review.
-
Distribution and pharmacology of alpha 6-containing nicotinic acetylcholine receptors analyzed with mutant mice.J Neurosci. 2002 Feb 15;22(4):1208-17. doi: 10.1523/JNEUROSCI.22-04-01208.2002. J Neurosci. 2002. PMID: 11850448 Free PMC article.
-
One GABA and two acetylcholine receptors function at the C. elegans neuromuscular junction.Nat Neurosci. 1999 Sep;2(9):791-7. doi: 10.1038/12160. Nat Neurosci. 1999. PMID: 10461217 Free PMC article.
References
-
- Alkondon M, Albuquerque EX. Diversity of nicotinic acetylcholine receptors in rat hippocampal neurons. I. Pharmacological and functional evidence for distinct structural subtypes. J Pharmacol Exp Ther. 1993;265:1455–1473. - PubMed
-
- Alkondon M, Reinhardt S, Lobron C, Hermsen B, Maelicke A, Albuquerque EX. Diversity of nicotinic acetylcholine receptors in rat hippocampal neurons. II. The rundown and inward rectification of agonist-elicited whole-cell currents and identification of receptor subunits by in situ hybridization. J Pharmacol Exp Ther. 1994;271:494–506. - PubMed
-
- Aubert I, Cecyre D, Gauthier S, Quirion R. Comparative ontogenic profile of cholinergic markers, including nicotinic and muscarinic receptors, in the rat brain. J Comp Neurol. 1996;369:31–55. - PubMed
-
- Badio B, Daly JW. Epibatidine, a potent analgetic and nicotinic agonist. Mol Pharmacol. 1994;45:563–569. - PubMed
-
- Benfenati F, Cimino M, Agnati LF, Fuxe K. Quantitative autoradiography of central neurotransmitter receptors: methodological and statistical aspects with special reference to computer-assisted image analysis. Acta Physiol Scand. 1986;128:129–146. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
