Multivesicular release at single functional synaptic sites in cerebellar stellate and basket cells
- PMID: 9614230
- PMCID: PMC6792676
- DOI: 10.1523/JNEUROSCI.18-12-04532.1998
Multivesicular release at single functional synaptic sites in cerebellar stellate and basket cells
Abstract
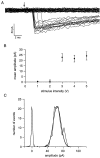
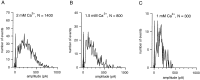
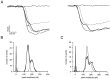
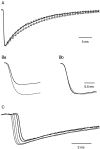
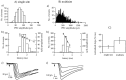
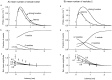
The purpose of the present work was to test the hypothesis that no more than one vesicle of transmitter can be liberated by an action potential at a single release site. Spontaneous and evoked IPSCs were recorded from interneurons in the molecular layer of cerebellar slices. Evoked IPSCs were obtained using either extracellular stimulation or paired recordings of presynaptic and postsynaptic neurons. Connections were identified as single-site synapses when evoked current amplitudes could be grouped into one peak that was well separated from the background noise. Peak amplitudes ranged from 30 to 298 pA. Reducing the release probability by lowering the external Ca2+ concentration or adding Cd2+ failed to reveal smaller quantal components. Some spontaneous IPSCs (1.4-2.4%) and IPSCs evoked at single-site synapses (2-6%) were followed within <5 msec by a secondary IPSC that could not be accounted for by random occurrence of background IPSCs. Nonlinear summation of closely timed events indicated that they involved activation of a common set of receptors and therefore that several vesicles could be released at the same release site by one action potential. An average receptor occupancy of 0.70 was calculated after single release events. At some single-site connections, two closely spaced amplitude peaks were resolved, presumably reflecting single and double vesicular release. Consistent with multivesicular release, kinetics of onset, decay, and latency were correlated to IPSC amplitude. We conclude that the one-site, one-vesicle hypothesis does not hold at interneuron-interneuron synapses.
Figures




References
-
- Auger C, Marty A. Heterogeneity of functional synaptic parameters among single release sites. Neuron. 1997;19:139–150. - PubMed
-
- Barbour B, Häusser M. Intersynaptic diffusion of neurotransmitter. Trends Neurosci. 1997;20:377–384. - PubMed
-
- Bekkers JM. Quantal analysis of synaptic transmission in the central nervous system. Curr Opin Neurobiol. 1994;4:360–365. - PubMed
-
- Clements JD. Quantal synaptic transmission? Nature. 1991;353:396. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
