Changes in mitochondrial function resulting from synaptic activity in the rat hippocampal slice
- PMID: 9614233
- PMCID: PMC6792701
- DOI: 10.1523/JNEUROSCI.18-12-04570.1998
Changes in mitochondrial function resulting from synaptic activity in the rat hippocampal slice
Abstract
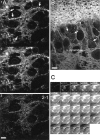
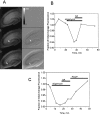
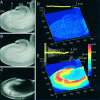
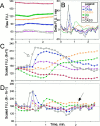
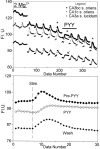
Digital imaging microfluorimetry was used to visualize changes in mitochondrial potential and intracellular Ca2+ concentration, [Ca2+]i, in thick slices of rat hippocampus. Electrical activity, especially stimulus train-induced bursting (STIB) activity, produced slow, prolonged changes in mitochondrial potential within hippocampal slices as revealed by fluorescence measurements with rhodamine dyes. Changes in mitochondrial potential showed both temporal and spatial correlations with the intensity of the electrical activity. Patterned changes in mitochondrial potential were observed to last from tens of seconds to minutes as the consequence of epileptiform discharges. STIB-associated elevations in [Ca2+]i were also prolonged and exhibited a spatial pattern similar to that of the mitochondrial depolarization. The mitochondrial depolarization was sensitive to TTX and glutamate receptor blockers ([Mg2+]o and CNQX or DNQX plus D-AP-5) and to the inhibition of glutamate release by activation of presynaptic NPY receptors. The monitoring of mitochondrial potential in slice preparations provides a new tool for mapping synaptic activity in the brain and for determining the roles of mitochondria in regulation of brain synaptic activity.
Figures






References
-
- Alagarsamy S, Lonart G, Johnson KM. Regulation of nitric oxide synthase activity in cortical slices by excitatory amino acids and calcium. J Neurosci Res. 1994;38:648–653. - PubMed
-
- Anderson WW, Lewis DV, Swartzwelder HS, Wilson WA. Magnesium-free medium activates seizure-like events in the rat hippocampal slice. Brain Res. 1986;398:215–219. - PubMed
-
- Anderson WW, Swartzwelder HS, Wilson WA. The NMDA receptor antagonist 2-amino-5-phosphonovalerate blocks stimulus train-induced epileptogenesis but not epileptiform bursting in the rat hippocampal slice. J Neurophysiol. 1987;57:1–21. - PubMed
-
- Andrew RD, MacVicar BA. Imaging cell volume changes and neuronal excitation in the hippocampal slice. Neuroscience. 1994;62:371–383. - PubMed
-
- Ankarcrona M, Dypdukt JM, Bonfoco E, Zhivotovsky B, Orrenius S, Lipton SA, Nicotera P. Glutamate-induced neuronal death: a succession of necrosis or apoptosis depending on mitochondrial function. Neuron. 1995;15:961–973. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
