Myosin Ibeta is located at tip link anchors in vestibular hair bundles
- PMID: 9614235
- PMCID: PMC6792677
- DOI: 10.1523/JNEUROSCI.18-12-04603.1998
Myosin Ibeta is located at tip link anchors in vestibular hair bundles
Abstract
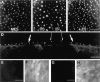
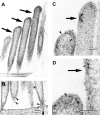
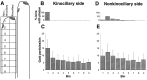
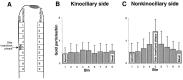
Recent studies have suggested that myosin Ibeta mediates the adaptation of mechanoelectrical transduction in vestibular hair cells. An important prediction of this hypothesis is that myosin Ibeta should be found in the side insertional plaque, an osmiophilic hair bundle structure that anchors tip links and is thought to house the adaptation motor. To determine whether myosin Ibeta was situated properly to perform adaptation, we used immunofluorescence and immunoelectron microscopy with the monoclonal antibody mT2 to examine the distribution of myosin Ibeta in hair bundles of the bullfrog utricle. Although utricular hair cells differ in their rates and extent of adaptation [Baird RA (1994) Comparative transduction mechanisms of hair cells in the bullfrog utriculus. II. Sensitivity and response dynamics to hair bundle displacement. J Neurophysiol 71:685-705.], myosin Ibeta was present in all hair bundles, regardless of adaptation kinetics. Confirming that, nevertheless, it was positioned properly to mediate adaptation, myosin Ibeta was found at significantly higher levels in the side insertional plaque. Myosin Ibeta was also present at elevated levels at the second tip link anchor of a hair bundle, the tip insertional plaque, found at the tip of a stereocilium. These data support myosin Ibeta as the adaptation motor and are consistent with the suggestion that the motor serves to restore tension applied to transduction channels to an optimal level, albeit with different kinetics in different cell types.
Figures




References
-
- Assad JA, Shepherd GMG, Corey DP. Tip-link integrity and mechanical transduction in vertebrate hair cells. Neuron. 1991;7:985–994. - PubMed
-
- Baird RA. Morphological and electrophysiological properties of hair cells in the bullfrog utriculus. Ann NY Acad Sci. 1992;656:12–26. - PubMed
-
- Baird RA. Comparative transduction mechanisms of hair cells in the bullfrog utriculus. I. Responses to intracellular current. J Neurophysiol. 1994a;71:666–684. - PubMed
-
- Baird RA. Comparative transduction mechanisms of hair cells in the bullfrog utriculus. II. Sensitivity and response dynamics to hair bundle displacement. J Neurophysiol. 1994b;71:685–705. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
