Connectivity and convergence of single corticostriatal axons
- PMID: 9614246
- PMCID: PMC6792707
- DOI: 10.1523/JNEUROSCI.18-12-04722.1998
Connectivity and convergence of single corticostriatal axons
Abstract
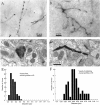
The distribution of synapses formed by corticostriatal neurons was measured to determine the average connectivity and degree of convergence of these neurons and to search for spatial inhomogeneities. Two kinds of axonal fields, focal and extended, and two striatal tissue compartments, the patch (striosome) and matrix, were analyzed separately. Electron microscopic examination revealed that both kinds of corticostriatal axons made synapses at varicosities that could be identified in the light microscope, and each varicosity made a single synapse. Thus, the distribution of varicosities was a good estimate of the spatial distribution of synapses. The distance between axonal varicosities was measured to determine the density of synaptic connections formed by one axon within the volume occupied by a striatal neuron. Intersynaptic distances were distributed exponentially, except that synapses were rarely located <4 microm apart. The mean distance between synapses was approximately 10 microm, so axons made a maximum of 40 synapses within the dendritic volume of a spiny neuron. There are approximately 2840 spiny neurons located within the volume of the dendrites of one spiny cell (Oorschot, 1996), so each axon must contact </=1.4% of all cells in its axonal arborization. Within the same volume there are approximately 30.5 million asymmetric synapses (Ingham et al., 1996), approximately half of which are cortical in origin. Thus, approximately 380,000 cortical axons innervate the volume of the dendritic tree of one spiny cell. Striatal neurons with totally overlapping dendritic volumes have few presynaptic cortical axons in common, and cortical cells with overlapping axons have few striatal target neurons in common. These results explain the absence of redundancy in the responses of neurons located near each other in the striatum.
Figures

Similar articles
-
Synaptic organization of the striatum.J Electron Microsc Tech. 1988 Nov;10(3):265-81. doi: 10.1002/jemt.1060100305. J Electron Microsc Tech. 1988. PMID: 3069970 Review.
-
Synaptic convergence of motor and somatosensory cortical afferents onto GABAergic interneurons in the rat striatum.J Neurosci. 2002 Sep 15;22(18):8158-69. doi: 10.1523/JNEUROSCI.22-18-08158.2002. J Neurosci. 2002. PMID: 12223570 Free PMC article.
-
Identification of synaptic terminals of thalamic or cortical origin in contact with distinct medium-size spiny neurons in the rat neostriatum.J Comp Neurol. 1988 Jan 22;267(4):455-71. doi: 10.1002/cne.902670402. J Comp Neurol. 1988. PMID: 3346370
-
A dopaminergic axon lattice in the striatum and its relationship with cortical and thalamic terminals.J Neurosci. 2008 Oct 29;28(44):11221-30. doi: 10.1523/JNEUROSCI.2780-08.2008. J Neurosci. 2008. PMID: 18971464 Free PMC article.
-
The underside of the cerebral cortex: layer V/VI spiny inverted neurons.J Anat. 2007 Aug;211(2):223-36. doi: 10.1111/j.1469-7580.2007.00779.x. Epub 2007 Jul 17. J Anat. 2007. PMID: 17635629 Free PMC article. Review.
Cited by
-
Oculomotor learning revisited: a model of reinforcement learning in the basal ganglia incorporating an efference copy of motor actions.Front Neural Circuits. 2012 Jun 27;6:38. doi: 10.3389/fncir.2012.00038. eCollection 2012. Front Neural Circuits. 2012. PMID: 22754501 Free PMC article.
-
Corticostriatal projections from rat barrel cortex have an anisotropic organization that correlates with vibrissal whisking behavior.J Neurosci. 1999 Dec 15;19(24):10908-22. doi: 10.1523/JNEUROSCI.19-24-10908.1999. J Neurosci. 1999. PMID: 10594072 Free PMC article.
-
Effects of motivational conflicts on visually elicited saccades in monkeys.Exp Brain Res. 2003 Oct;152(3):361-7. doi: 10.1007/s00221-003-1555-9. Epub 2003 Aug 1. Exp Brain Res. 2003. PMID: 12904939
-
Cholinergic neurons in the pedunculopontine tegmental nucleus modulate breathing in rats by direct projections to the retrotrapezoid nucleus.J Physiol. 2019 Apr;597(7):1919-1934. doi: 10.1113/JP277617. Epub 2019 Mar 1. J Physiol. 2019. PMID: 30724347 Free PMC article.
-
Reconstructing the three-dimensional GABAergic microcircuit of the striatum.PLoS Comput Biol. 2010 Nov 24;6(11):e1001011. doi: 10.1371/journal.pcbi.1001011. PLoS Comput Biol. 2010. PMID: 21124867 Free PMC article.
References
-
- Abeles M. Corticonics, pp 67–72. Cambridge UP; Cambridge, UK: 1991.
-
- Alexander GE, Crutcher MD. Preparation for movement: neural representations of intended direction in three motor areas of the monkey. J Neurophysiol. 1990;64:133–150. - PubMed
-
- Alexander GE, DeLong MR. Microstimulation of the primate neostriatum. I. Physiological properties of striatal microexcitable zones. J Neurophysiol. 1985a;53:1401–1416. - PubMed
-
- Alexander GE, DeLong MR. Microstimulation of the primate neostriatum. II. Somatotopic organization of striatal microexcitable zones and their relation to neuronal response properties. J Neurophysiol. 1985b;53:1417–1430. - PubMed
-
- Alexander GE, DeLong MR, Crutcher MD. Do cortical and basal ganglionic motor areas use “motor programs” to control movement? Behav Brain Sci. 1992;15:656–665.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
