Apoptosis is a major mode of cell death caused by ischaemia and ischaemia/reperfusion injury to the rat intestinal epithelium
- PMID: 9616316
- PMCID: PMC1727054
- DOI: 10.1136/gut.42.4.530
Apoptosis is a major mode of cell death caused by ischaemia and ischaemia/reperfusion injury to the rat intestinal epithelium
Abstract
Background and aims: Injuries caused by ischaemia and ischaemia/reperfusion in the small intestine have been widely accepted as resulting in necrosis. The aim of this study was to ascertain whether apoptosis also occurs.
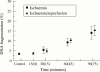
Methods: Intestinal epithelium from rats subjected to ischaemia (15-90 minutes) and ischaemia/reperfusion (15 minutes ischaemia followed by 15-75 minutes of reperfusion) was studied using histological, immunohistochemical, and molecular biological methods as well as FACS.
Results: Mucosal injury was induced by both ischaemia and ischaemia/reperfusion. Detachment of epithelial cells from the villous stroma was an early morphological change indicating mucosal injury. More than 80% of the detached cells exhibited characteristic morphological features of apoptosis (condensation of chromatin and nuclear fragmentation). The remainder demonstrated necrotic features. The apoptotic cells eventually underwent spontaneous degeneration with membrane rupture, a process morphologically identical to necrosis. DNA fragmentation was also confirmed by immunohistochemical methods and agarose gel electrophoresis.
Conclusion: Apoptosis is a major mode of cell death in the destruction of rat small intestinal epithelial cells induced by ischaemia and ischaemia/reperfusion injury. Disruption of epithelial cell-matrix interactions ("anoikis") may play an important part in induction of apoptosis in detached enterocytes.
Figures





References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
