Signalling in the yeasts: an informational cascade with links to the filamentous fungi
- PMID: 9618441
- PMCID: PMC98914
- DOI: 10.1128/MMBR.62.2.249-274.1998
Signalling in the yeasts: an informational cascade with links to the filamentous fungi
Abstract
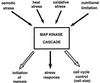
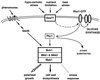
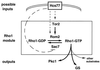
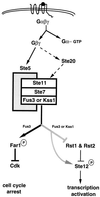
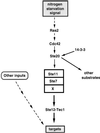
All cells, from bacteria and yeasts to mammalian cells, respond to cues from their environment. A variety of mechanisms exist for the transduction of these external signals to the interior of the cell, resulting in altered patterns of protein activity. Eukaryotic cells commonly transduce external cues via a conserved module composed of three protein kinases, the mitogen-activated protein kinase (MAPK) cascade. This module can then activate substrates, some of which include transcriptional activators. Multiple MAPK signalling pathways coexist in a cell. This review considers different MAPK cascade signalling pathways that govern several aspects of the life cycle of budding and fission yeasts: conjugation and meiosis by the pheromone response pathway, stress response by the high-osmolarity sensing pathway, cell wall biosynthesis in response to activation of the low-osmolarity and heat-sensing pathway, and pseudohyphal growth in response to activation of a subset of the components of the pheromone response pathway. Because the MAPK cascade components are highly conserved, a key question in studies of these pathways is the mechanism by which specificity of response is achieved. Several other issues to be addressed in this review concern the nature of the receptors used to sense the external signals and the mechanism by which the receptors communicate with other components leading to activation of the MAPK cascade. Recently, it has become apparent that MAPK cascades are important in governing the pathogenicity of filamentous fungi.
Figures






References
-
- Aiba H, Yamada H, Ohmiya R, Mizuno T. The osmoinducible gpd1+ gene is a target of the signaling pathway involving Wis1 MAP-kinase kinase in fission yeast. FEBS Lett. 1995;376:199–201. - PubMed
-
- Albertyn J, Hohmann S, Thevelein J M, Prior B A. GPD1, which encodes glycerol-3-phosphate dehydrogenase, is essential for growth under osmotic stress in Saccharomyces cerevisiae, and its expression is regulated by the high-osmolarity glycerol response pathway. Mol Cell Biol. 1994;14:4135–4144. - PMC - PubMed
-
- Aono T, Yanah H, Miki F, Davey J, Shimoda C. Mating pheromone-induced expression of the mat1-Pm gene of Schizosaccharomyces pombe: identification of signalling components and characterization of upstream controlling elements. Yeast. 1994;10:757–770. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

