Interacting helical faces of subunits a and c in the F1Fo ATP synthase of Escherichia coli defined by disulfide cross-linking
- PMID: 9618459
- PMCID: PMC22573
- DOI: 10.1073/pnas.95.12.6607
Interacting helical faces of subunits a and c in the F1Fo ATP synthase of Escherichia coli defined by disulfide cross-linking
Abstract
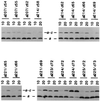
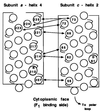
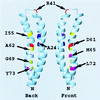
Subunits a and c of Fo are thought to cooperatively catalyze proton translocation during ATP synthesis by the Escherichia coli F1Fo ATP synthase. Optimizing mutations in subunit a at residues A217, I221, and L224 improves the partial function of the cA24D/cD61G double mutant and, on this basis, these three residues were proposed to lie on one face of a transmembrane helix of subunit a, which then interacted with the transmembrane helix of subunit c anchoring the essential aspartyl group. To test this model, in the present work Cys residues were introduced into the second transmembrane helix of subunit c and the predicted fourth transmembrane helix of subunit a. After treating the membrane vesicles of these mutants with Cu(1, 10-phenanthroline)2SO4 at 0 degrees, 10 degrees, or 20 degreesC, strong a-c dimer formation was observed at all three temperatures in membranes of 7 of the 65 double mutants constructed, i.e., in the aS207C/cI55C, aN214C/cA62C, aN214C/cM65C, aI221C/cG69C, aI223C/cL72C, aL224C/cY73C, and aI225C/cY73C double mutant proteins. The pattern of cross-linking aligns the helices in a parallel fashion over a span of 19 residues with the aN214C residue lying close to the cA62C and cM65C residues in the middle of the membrane. Lesser a-c dimer formation was observed in nine other double mutants after treatment at 20 degreesC in a pattern generally supporting that indicated by the seven landmark residues cited above. Cross-link formation was not observed between helix-1 of subunit c and helix-4 of subunit a in 19 additional combinations of doubly Cys-substituted proteins. These results provide direct chemical evidence that helix-2 of subunit c and helix-4 of subunit a pack close enough to each other in the membrane to interact during function. The proximity of helices supports the possibility of an interaction between Arg210 in helix-4 of subunit a and Asp61 in helix-2 of subunit c during proton translocation, as has been suggested previously.
Figures



References
-
- Boyer P D. Annu Rev Biochem. 1997;66:717–749. - PubMed
-
- Deckers-Hebestreit G, Altendorf K. Annu Rev Microbiol. 1996;50:791–824. - PubMed
-
- Fillingame R H. J Exp Biol. 1997;200:217–224. - PubMed
-
- Fillingame R H. The Bacteria. Vol. 12. New York: Academic; 1990. pp. 345–391.
-
- Abrahams J P, Leslie A G, Lutter R, Walker J E. Nature (London) 1994;370:621–628. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

