Lignin monomer composition is determined by the expression of a cytochrome P450-dependent monooxygenase in Arabidopsis
- PMID: 9618461
- PMCID: PMC22575
- DOI: 10.1073/pnas.95.12.6619
Lignin monomer composition is determined by the expression of a cytochrome P450-dependent monooxygenase in Arabidopsis
Abstract
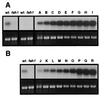
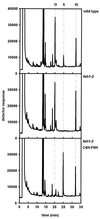
The phenylpropanoid pathway provides precursors for the biosynthesis of soluble secondary metabolites and lignin in plants. Ferulate-5-hydroxylase (F5H) catalyzes an irreversible hydroxylation step in this pathway that diverts ferulic acid away from guaiacyl lignin biosynthesis and toward sinapic acid and syringyl lignin. This fact led us to postulate that F5H was a potential regulatory step in the determination of lignin monomer composition. To test this hypothesis, we have used Arabidopsis to examine the impact of F5H overexpression. Arabidopsis is a useful model system in which to study lignification because in wild-type plants, guaiacyl and syringyl lignins are deposited in a tissue-specific fashion, while the F5H-deficient fah1 mutant accumulates only guaiacyl lignin. Here we show that ectopic overexpression of F5H in Arabidopsis abolishes tissue-specific lignin monomer accumulation. Surprisingly, overexpression of F5H under the control of the lignification-associated cinnamate-4-hydroxylase promoter, but not the commonly employed cauliflower mosaic virus 35S promoter, generates a lignin that is almost entirely comprised of syringylpropane units. These experiments demonstrate that modification of F5H expression may enable engineering of lignin monomer composition in agronomically important plant species.
Figures



References
-
- Higuchi T. In: Plant Carbohydrates II. Tanner W, Loewus F A, editors. New York: Springer; 1981. pp. 194–224.
-
- Lewis N G, Yamamoto E. Annu Rev Plant Physiol Plant Mol Biol. 1990;41:455–496. - PubMed
-
- Mizutani M, Ward E, Ohta D, Ryals J, Sato R. Biochem Biophys Res Commun. 1993;190:875–880. - PubMed
-
- Grand C. FEBS Lett. 1984;169:7–11.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

