The RIIbeta regulatory subunit of protein kinase A binds to cAMP response element: an alternative cAMP signaling pathway
- PMID: 9618473
- PMCID: PMC22599
- DOI: 10.1073/pnas.95.12.6687
The RIIbeta regulatory subunit of protein kinase A binds to cAMP response element: an alternative cAMP signaling pathway
Abstract
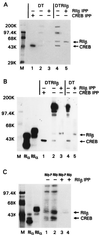
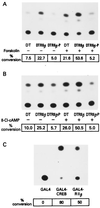
cAMP, through the activation of cAMP-dependent protein kinase (PKA), is involved in transcriptional regulation. In eukaryotic cells, cAMP is not considered to alter the binding affinity of CREB/ATF to cAMP-responsive element (CRE) but to induce serine phosphorylation and consequent increase in transcriptional activity. In contrast, in prokaryotic cells, cAMP enhances the DNA binding of the catabolite repressor protein to regulate the transcription of several operons. The structural similarity of the cAMP binding sites in catabolite repressor protein and regulatory subunit of PKA type II (RII) suggested the possibility of a similar role for RII in eukaryotic gene regulation. Herein we report that RIIbeta subunit of PKA is a transcription factor capable of interacting physically and functionally with a CRE. In contrast to CREB/ATF, the binding of RIIbeta to a CRE was enhanced by cAMP, and in addition, RIIbeta exhibited transcriptional activity as a Gal4-RIIbeta fusion protein. These experiments identify RIIbeta as a component of an alternative pathway for regulation of CRE-directed transcription in eukaryotic cells.
Figures


Similar articles
-
Protein kinase A regulatory subunit type II beta directly interacts with and suppresses CREB transcriptional activity in activated T cells.J Immunol. 2003 Oct 1;171(7):3636-44. doi: 10.4049/jimmunol.171.7.3636. J Immunol. 2003. PMID: 14500661
-
The differential response of protein kinase A to cyclic AMP in discrete brain areas correlates with the abundance of regulatory subunit II.J Neurochem. 1996 Apr;66(4):1752-61. doi: 10.1046/j.1471-4159.1996.66041752.x. J Neurochem. 1996. PMID: 8627334
-
A-kinase anchor protein 75 increases the rate and magnitude of cAMP signaling to the nucleus.Curr Biol. 1997 Dec 1;7(12):1011-4. doi: 10.1016/s0960-9822(06)00424-6. Curr Biol. 1997. PMID: 9382844
-
Chemoprevention with protein kinase A RIalpha antisense in DMBA-mammary carcinogenesis.Ann N Y Acad Sci. 2005 Nov;1058:255-64. doi: 10.1196/annals.1359.038. Ann N Y Acad Sci. 2005. PMID: 16394142 Review.
-
Protein kinase A: regulation and receptor-mediated delivery of antisense oligonucleotides and cytotoxic drugs.Ann N Y Acad Sci. 2002 Jun;968:158-72. doi: 10.1111/j.1749-6632.2002.tb04334.x. Ann N Y Acad Sci. 2002. PMID: 12119275 Review.
Cited by
-
Epac, not PKA catalytic subunit, is required for 3T3-L1 preadipocyte differentiation.Front Biosci (Elite Ed). 2010 Jan 1;2(2):392-8. doi: 10.2741/e99. Front Biosci (Elite Ed). 2010. PMID: 20036887 Free PMC article.
-
Adenylyl cyclase-cyclicAMP signaling in mood disorders: role of the crucial phosphorylating enzyme protein kinase A.Neuropsychiatr Dis Treat. 2008 Feb;4(1):161-76. doi: 10.2147/ndt.s2380. Neuropsychiatr Dis Treat. 2008. PMID: 18728821 Free PMC article.
References
-
- Krebs E G, Beavo J A. Annu Rev Biochem. 1979;48:923–939. - PubMed
-
- Beebe S J, Corbin J D. In: The Enzymes: Control by Phosphorylation. Boyer P D, Krebs E G, editors. Vol. 17. New York: Academic; 1986. pp. 43–111.
-
- McKnight G S, Clegg C H, Uhler M D, Chrivia J C, Cadd G G, Correll L A, Otten A D. Recent Prog Horm Res. 1988;44:307–355. - PubMed
-
- Cho-Chung Y S. Cancer Res. 1990;50:7093–7100. - PubMed
-
- Lohmann S M, Walter U. In: Advances in Cyclic Nucleotide and Protein Phosphorylation Research. Greengard P, Robinson G A, editors. 17A. New York: Raven; 1984. pp. 63–117. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

