p21(WAF1) is required for butyrate-mediated growth inhibition of human colon cancer cells
- PMID: 9618491
- PMCID: PMC22637
- DOI: 10.1073/pnas.95.12.6791
p21(WAF1) is required for butyrate-mediated growth inhibition of human colon cancer cells
Abstract
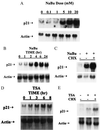
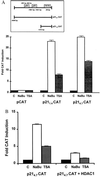
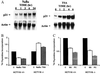
A diet high in fiber is associated with a decreased incidence and growth of colon cancers. Butyrate, a four-carbon short-chain fatty acid product of fiber fermentation within the colon, appears to mediate these salutary effects. We sought to determine the molecular mechanism by which butyrate mediates growth inhibition of colonic cancer cells and thereby to elucidate the molecular link between a high-fiber diet and the arrest of colon carcinogenesis. We show that concomitant with growth arrest, butyrate induces p21 mRNA expression in an immediate-early fashion, through transactivation of a promoter cis-element(s) located within 1.4 kb of the transcriptional start site, independent of p53 binding. Studies using the specific histone hyperacetylating agent, trichostatin A, and histone deacetylase 1 indicate that growth arrest and p21 induction occur through a mechanism involving histone hyperacetylation. We show the critical importance of p21 in butyrate-mediated growth arrest by first confirming that stable overexpression of the p21 gene is able to cause growth arrest in the human colon carcinoma cell line, HT-29. Furthermore, using p21-deleted HCT116 human colon carcinoma cells, we provide convincing evidence that p21 is required for growth arrest to occur in response to histone hyperacetylation, but not for serum starvation nor postconfluent growth. Thus, p21 appears to be a critical effector of butyrate-induced growth arrest in colonic cancer cells, and may be an important molecular link between a high-fiber diet and the prevention of colon carcinogenesis.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

