Static and dynamic lengths of neutrophil microvilli
- PMID: 9618492
- PMCID: PMC22640
- DOI: 10.1073/pnas.95.12.6797
Static and dynamic lengths of neutrophil microvilli
Abstract
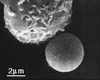
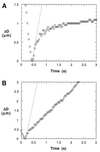
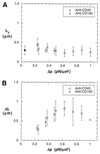
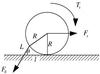
Containing most of the L-selectin and P-selectin glycoprotein ligand-1 (PSGL-1) on their tips, microvilli are believed to promote the initial arrest of neutrophils on endothelium. At the rolling stage following arrest, the lifetimes of the involved molecular bonds depend on the pulling force imposed by the shear stress of blood flow. With two different methods, electron microscopy and micropipette manipulation, we have obtained two comparable neutrophil microvillus lengths, both approximately 0.3 microm in average. We have found also that, under a pulling force, a microvillus can be extended (microvillus extension) or a long thin membrane cylinder (a tether) can be formed from it (tether formation). If the force is </=34 pN (+/- 3 pN), the length of the microvillus will be extended; if the force is >61 pN (+/- 5 pN), a tether will be formed from the microvillus at a constant velocity, which depends linearly on the force. When the force is between 34 pN and 61 pN (transition zone), the degree of association between membrane and cytoskeleton in individual microvilli will dictate whether microvillus extension or tether formation occurs. When a microvillus is extended, it acts like a spring with a spring constant of approximately 43 pN/microm. In contrast to a rigid or nonextendible microvillus, both microvillus extension and tether formation can decrease the pulling force imposed on the adhesive bonds, and thus prolonging the persistence of the bonds at high physiological shear stresses.
Figures

Similar articles
-
Direct observation of membrane tethers formed during neutrophil attachment to platelets or P-selectin under physiological flow.J Cell Biol. 2000 May 1;149(3):719-30. doi: 10.1083/jcb.149.3.719. J Cell Biol. 2000. PMID: 10791984 Free PMC article.
-
Comparison of PSGL-1 microbead and neutrophil rolling: microvillus elongation stabilizes P-selectin bond clusters.Biophys J. 2002 Apr;82(4):1835-47. doi: 10.1016/S0006-3495(02)75534-3. Biophys J. 2002. PMID: 11916843 Free PMC article.
-
Dynamics of Microvillus Extension and Tether Formation in Rolling Leukocytes.Cell Mol Bioeng. 2009;2(2):207-217. doi: 10.1007/s12195-009-0063-9. Cell Mol Bioeng. 2009. PMID: 20046963 Free PMC article.
-
Biomechanics of leukocyte rolling.Biorheology. 2011;48(1):1-35. doi: 10.3233/BIR-2011-0579. Biorheology. 2011. PMID: 21515934 Free PMC article. Review.
-
Simulation of cell rolling and adhesion on surfaces in shear flow. Microvilli-coated hard spheres with adhesive springs.Cell Biophys. 1991 Apr;18(2):145-82. doi: 10.1007/BF02989811. Cell Biophys. 1991. PMID: 1726526 Review.
Cited by
-
Tangential tether extraction and spontaneous tether retraction of human neutrophils.Biophys J. 2012 Dec 5;103(11):2257-64. doi: 10.1016/j.bpj.2012.10.018. Biophys J. 2012. PMID: 23283224 Free PMC article.
-
Distinct molecular and cellular contributions to stabilizing selectin-mediated rolling under flow.J Cell Biol. 2002 Aug 19;158(4):787-99. doi: 10.1083/jcb.200204041. Epub 2002 Aug 12. J Cell Biol. 2002. PMID: 12177042 Free PMC article.
-
Mechanics of transient platelet adhesion to von Willebrand factor under flow.Biophys J. 2005 Feb;88(2):1432-43. doi: 10.1529/biophysj.104.047001. Epub 2004 Nov 8. Biophys J. 2005. PMID: 15533923 Free PMC article.
-
Direct observation of membrane tethers formed during neutrophil attachment to platelets or P-selectin under physiological flow.J Cell Biol. 2000 May 1;149(3):719-30. doi: 10.1083/jcb.149.3.719. J Cell Biol. 2000. PMID: 10791984 Free PMC article.
-
A Novel Technique of Quantifying Flexural Stiffness of Rod-Like Structures.Cell Mol Bioeng. 2008 Mar 18;1(1):75-83. doi: 10.1007/s12195-008-0012-z. Cell Mol Bioeng. 2008. PMID: 20333317 Free PMC article.
References
-
- Bruehl R E, Springer T A, Bainton D F. J Histochem Cytochem. 1996;44:835–844. - PubMed
-
- Bruehl R E, Moore K L, Lorant D E, Borregaard N, Zimmerman G A, McEver R P, Bainton D F. J Leukocyte Biol. 1997;61:489–499. - PubMed
-
- Erlandsen S L, Hasslen S R, Nelson R D. J Histochem Cytochem. 1993;41:327–333. - PubMed
-
- Finger E B, Bruehl R E, Bainton D F, Springer T A. J Immunol. 1996;157:5085–5096. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

