Cross-lineage expression of Ig-beta (B29) in thymocytes: positive and negative gene regulation to establish T cell identity
- PMID: 9618498
- PMCID: PMC22652
- DOI: 10.1073/pnas.95.12.6831
Cross-lineage expression of Ig-beta (B29) in thymocytes: positive and negative gene regulation to establish T cell identity
Abstract
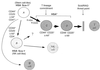
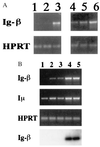
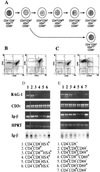
Developmental commitment involves activation of lineage-specific genes, stabilization of a lineage-specific gene expression program, and permanent inhibition of inappropriate characteristics. To determine how these processes are coordinated in early T cell development, the expression of T and B lineage-specific genes was assessed in staged subsets of immature thymocytes. T lineage characteristics are acquired sequentially, with germ-line T cell antigen receptor-beta transcripts detected very early, followed by CD3epsilon and terminal deoxynucleotidyl transferase, then pTalpha, and finally RAG1. Only RAG1 expression coincides with commitment. Thus, much T lineage gene expression precedes commitment and does not depend on it. Early in the course of commitment to the T lineage, thymocytes lose the ability to develop into B cells. To understand how this occurs, we also examined expression of well defined B lineage-specific genes. Although lambda5 and Ig-alpha are not expressed, the mu 0 and I mu transcripts from the unrearranged IgH locus are expressed early, in distinct patterns, then repressed just before RAG1 expression. By contrast, RNA encoding the B cell receptor component Ig-beta was found to be transcribed in all immature thymocyte subpopulations and throughout most thymocyte differentiation. Ig-beta expression is down-regulated only during positive selection of CD4(+)CD8(-) cells. Thus several key participants in the B cell developmental program are expressed in non-B lineage-committed cells, and one is maintained even through commitment to an alternative lineage, and repressed only after extensive T lineage differentiation. The results show that transcriptional activation of "lymphocyte-specific" genes can occur in uncommitted precursors, and that T lineage commitment is a composite of distinct positive and negative regulatory events.
Figures

Similar articles
-
Fidelity and infidelity in commitment to B-lymphocyte lineage development.Immunol Rev. 2000 Jun;175:104-11. Immunol Rev. 2000. PMID: 10933595 Review.
-
Progression of T cell lineage restriction in the earliest subpopulation of murine adult thymus visualized by the expression of lck proximal promoter activity.Int Immunol. 2001 Jan;13(1):105-17. doi: 10.1093/intimm/13.1.105. Int Immunol. 2001. PMID: 11133839
-
Precocious expression of T cell functional response genes in vivo in primitive thymocytes before T lineage commitment.Int Immunol. 1998 Nov;10(11):1623-35. doi: 10.1093/intimm/10.11.1623. Int Immunol. 1998. PMID: 9846691
-
Emergence of T, B, and myeloid lineage-committed as well as multipotent hemopoietic progenitors in the aorta-gonad-mesonephros region of day 10 fetuses of the mouse.J Immunol. 1999 Nov 1;163(9):4788-95. J Immunol. 1999. PMID: 10528178
-
Transcriptional regulation of lymphocyte lineage commitment.Bioessays. 1999 Sep;21(9):726-42. doi: 10.1002/(SICI)1521-1878(199909)21:9<726::AID-BIES4>3.0.CO;2-S. Bioessays. 1999. PMID: 10462413 Review.
Cited by
-
B cell-restricted human mb-1 gene: expression, function, and lineage infidelity.Immunol Res. 2002;26(1-3):35-43. doi: 10.1385/ir:26:1-3:035. Immunol Res. 2002. PMID: 12403343 Review.
-
A conserved sequence upstream of the B29 (Ig beta, CD79b) gene interacts with YY1.Mol Biol Rep. 2004 Mar;31(1):1-11. doi: 10.1023/b:mole.0000013489.04734.5e. Mol Biol Rep. 2004. PMID: 15040449
-
Pax5 induces V-to-DJ rearrangements and locus contraction of the immunoglobulin heavy-chain gene.Genes Dev. 2004 Feb 15;18(4):411-22. doi: 10.1101/gad.291504. Genes Dev. 2004. PMID: 15004008 Free PMC article.
-
B29 gene silencing in pituitary cells is regulated by its 3' enhancer.J Mol Biol. 2006 Sep 15;362(2):173-83. doi: 10.1016/j.jmb.2006.07.046. Epub 2006 Jul 28. J Mol Biol. 2006. PMID: 16920149 Free PMC article.
-
Progression of regulatory gene expression states in fetal and adult pro-T-cell development.Immunol Rev. 2006 Feb;209:212-36. doi: 10.1111/j.0105-2896.2006.00355.x. Immunol Rev. 2006. PMID: 16448545 Free PMC article. Review.
References
-
- Kee B L, Paige C J. Intl Rev Cytol. 1995;157:129–179. - PubMed
-
- Lin H, Grosschedl R. Nature (London) 1995;376:263–267. - PubMed
-
- Nuez B, Michalovich D, Bygrave A, Ploemacher R, Grosveld F. Nature (London) 1995;375:316–318. - PubMed
-
- Olson M C, Scott E W, Hack A A, Su G H, Tenen D G, Singh H, Simon M C. Immunity. 1995;3:703–714. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

