Nucleolar localization of the Werner syndrome protein in human cells
- PMID: 9618508
- PMCID: PMC22674
- DOI: 10.1073/pnas.95.12.6887
Nucleolar localization of the Werner syndrome protein in human cells
Abstract
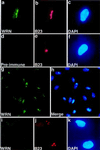
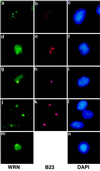
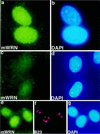
Werner Syndrome (WS) is a human genetic disorder with many features of premature aging. The gene defective in WS (WRN) has been cloned and encodes a protein homologous to several helicases, including Escherichia coli RecQ, the human Bloom syndrome protein (BLM), and Saccharomyces cerevisiae Sgs1p. To better define the function of WRN protein we have determined its subcellular localization. Indirect immunofluorescence using polyclonal anti-human WRN shows a predominant nucleolar localization. Studies of WRN mutant cells lines confirmed the specificity of antibody recognition. No difference was seen in the subcellular localization of the WRN protein in a variety of normal and transformed human cell lines, including both carcinomas and sarcomas. The nucleolar localization of human WRN protein was supported by the finding that upon biochemical subcellular fractionation, WRN protein is present in an increased concentration in a subnuclear fraction enriched for nucleolar proteins. We have also determined the subcellular localization of the mouse WRN homologue (mWRN). In contrast to human WRN protein, mWRN protein is present diffusely throughout the nucleus. Understanding the function of WRN in these organisms of vastly differing lifespan may yield new insights into the mechanisms of lifespan determination.
Figures


References
-
- Salk D. Hum Genet. 1982;62:1–5. - PubMed
-
- Epstein C J, Martin G M, Schultz A L, Motulsky A G. Medicine. 1966;45:177–221. - PubMed
-
- Martin G M, Sprague C A, Epstein C J. Lab Invest. 1970;23:86–91. - PubMed
-
- Holliday R, Thompson K V A, Huschtscha L I, Rattan S I S, Sedgwick S G, Spanos A. In: Werner’s Syndrome and Human Aging. Salk D, Fujiwara Y, Martin G M, editors. Vol. 190. New York: Plenum; 1982. pp. 331–339. - PubMed
-
- Salk D, Bryant E, Hoehn H, Johnston P, Martin G M. In: Werner’s Syndrome and Human Aging. Salk D, Fujiwara Y, Martin G M, editors. Vol. 190. New York: Plenum; 1982. pp. 305–312.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

