Aberrant intracellular localization of Varicella-Zoster virus regulatory proteins during latency
- PMID: 9618542
- PMCID: PMC22745
- DOI: 10.1073/pnas.95.12.7080
Aberrant intracellular localization of Varicella-Zoster virus regulatory proteins during latency
Abstract
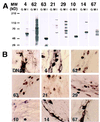
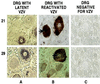
Varicella-Zoster virus (VZV) is a herpesvirus that becomes latent in sensory neurons after primary infection (chickenpox) and subsequently may reactivate to cause zoster. The mechanism by which this virus maintains latency, and the factors involved, are poorly understood. Here we demonstrate, by immunohistochemical analysis of ganglia obtained at autopsy from seropositive patients without clinical symptoms of VZV infection that viral regulatory proteins are present in latently infected neurons. These proteins, which localize to the nucleus of cells during lytic infection, predominantly are detected in the cytoplasm of latently infected neurons. The restriction of regulatory proteins from the nucleus of latently infected neurons might interrupt the cascade of virus gene expression that leads to a productive infection. Our findings raise the possibility that VZV has developed a novel mechanism for maintenance of latency that contrasts with the transcriptional repression that is associated with latency of herpes simplex virus, the prototypic alpha herpesvirus.
Figures



References
-
- Garcia-Blanco M A, Cullen B R. Science. 1991;254:815–820. - PubMed
-
- Ragozzino M W, Melton L J, 3rd, Kurland L T, Chu C P, Perry H O. Medicine (Baltimore) 1982;51:310–316. - PubMed
-
- Straus S E. J Am Med Assoc. 1993;269:1836–1839. - PubMed
-
- Hyman R W, Ecker J R, Tenser R B. Lancet. 1983;2:814–816. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

