MgATP activates the beta cell KATP channel by interaction with its SUR1 subunit
- PMID: 9618560
- PMCID: PMC22779
- DOI: 10.1073/pnas.95.12.7185
MgATP activates the beta cell KATP channel by interaction with its SUR1 subunit
Abstract
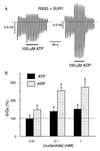
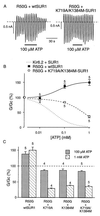
ATP-sensitive potassium (KATP) channels in the pancreatic beta cell membrane mediate insulin release in response to elevation of plasma glucose levels. They are open at rest but close in response to glucose metabolism, producing a depolarization that stimulates Ca2+ influx and exocytosis. Metabolic regulation of KATP channel activity currently is believed to be mediated by changes in the intracellular concentrations of ATP and MgADP, which inhibit and activate the channel, respectively. The beta cell KATP channel is a complex of four Kir6.2 pore-forming subunits and four SUR1 regulatory subunits: Kir6.2 mediates channel inhibition by ATP, whereas the potentiatory action of MgADP involves the nucleotide-binding domains (NBDs) of SUR1. We show here that MgATP (like MgADP) is able to stimulate KATP channel activity, but that this effect normally is masked by the potent inhibitory effect of the nucleotide. Mg2+ caused an apparent reduction in the inhibitory action of ATP on wild-type KATP channels, and MgATP actually activated KATP channels containing a mutation in the Kir6.2 subunit that impairs nucleotide inhibition (R50G). Both of these effects were abolished when mutations were made in the NBDs of SUR1 that are predicted to abolish MgATP binding and/or hydrolysis (D853N, D1505N, K719A, or K1384M). These results suggest that, like MgADP, MgATP stimulates KATP channel activity by interaction with the NBDs of SUR1. Further support for this idea is that the ATP sensitivity of a truncated form of Kir6.2, which shows functional expression in the absence of SUR1, is unaffected by Mg2+.
Figures
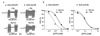

Similar articles
-
The Kir6.2-F333I mutation differentially modulates KATP channels composed of SUR1 or SUR2 subunits.J Physiol. 2007 Jun 15;581(Pt 3):1259-69. doi: 10.1113/jphysiol.2007.130211. Epub 2007 Mar 29. J Physiol. 2007. PMID: 17395632 Free PMC article.
-
A universally conserved residue in the SUR1 subunit of the KATP channel is essential for translating nucleotide binding at SUR1 into channel opening.J Physiol. 2012 Oct 15;590(20):5025-36. doi: 10.1113/jphysiol.2012.236075. Epub 2012 Jul 16. J Physiol. 2012. PMID: 22802590 Free PMC article.
-
Role of the C-terminus of SUR in the differential regulation of β-cell and cardiac KATP channels by MgADP and metabolism.J Physiol. 2018 Dec;596(24):6205-6217. doi: 10.1113/JP276708. Epub 2018 Oct 14. J Physiol. 2018. PMID: 30179258 Free PMC article.
-
Molecular biology of adenosine triphosphate-sensitive potassium channels.Endocr Rev. 1999 Apr;20(2):101-35. doi: 10.1210/edrv.20.2.0361. Endocr Rev. 1999. PMID: 10204114 Review.
-
ATP-sensitive potassium channels: a model of heteromultimeric potassium channel/receptor assemblies.Annu Rev Physiol. 1999;61:337-62. doi: 10.1146/annurev.physiol.61.1.337. Annu Rev Physiol. 1999. PMID: 10099692 Review.
Cited by
-
A gating mutation at the internal mouth of the Kir6.2 pore is associated with DEND syndrome.EMBO Rep. 2005 May;6(5):470-5. doi: 10.1038/sj.embor.7400393. EMBO Rep. 2005. PMID: 15864298 Free PMC article.
-
Characterization of two novel forms of the rat sulphonylurea receptor SUR1A2 and SUR1BDelta31.Br J Pharmacol. 2002 Sep;137(1):98-106. doi: 10.1038/sj.bjp.0704836. Br J Pharmacol. 2002. PMID: 12183335 Free PMC article.
-
Pyridine nucleotide regulation of the KATP channel Kir6.2/SUR1 expressed in Xenopus oocytes.J Physiol. 2003 Jul 15;550(Pt 2):357-63. doi: 10.1113/jphysiol.2003.041715. Epub 2003 May 23. J Physiol. 2003. PMID: 12766240 Free PMC article.
-
ATP binding without hydrolysis switches sulfonylurea receptor 1 (SUR1) to outward-facing conformations that activate KATP channels.J Biol Chem. 2019 Mar 8;294(10):3707-3719. doi: 10.1074/jbc.RA118.005236. Epub 2018 Dec 26. J Biol Chem. 2019. PMID: 30587573 Free PMC article.
-
Incomplete dissociation of glibenclamide from wild-type and mutant pancreatic K ATP channels limits their recovery from inhibition.Br J Pharmacol. 2009 Jan;156(2):354-61. doi: 10.1111/j.1476-5381.2008.00005.x. Epub 2009 Jan 13. Br J Pharmacol. 2009. PMID: 19154434 Free PMC article.
References
-
- Ashcroft, F. M. & Gribble, F. M. (1998) Trends Neurosci., in press. - PubMed
-
- Ashcroft F M, Ashcroft S J H. Cell Signalling. 1990;2:197–214. - PubMed
-
- Nichols C G, Lederer W J. Am J Physiol. 1991;261:H1675–H1686. - PubMed
-
- Bokvist K, Ämmälä C, Ashcroft F M, Berggren P O, Larsson O, Rorsman P. Proc R Soc London Ser B. 1991;243:139–144. - PubMed
-
- Tucker S J, Gribble F M, Zhao C, Trapp S, Ashcroft F M. Nature (London) 1997;378:179–183. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

