The tobacco wounding-activated mitogen-activated protein kinase is encoded by SIPK
- PMID: 9618567
- PMCID: PMC22786
- DOI: 10.1073/pnas.95.12.7225
The tobacco wounding-activated mitogen-activated protein kinase is encoded by SIPK
Abstract
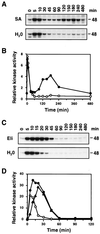
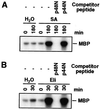
It has been demonstrated that both salicylic acid and fungal elicitors activate a 48-kDa mitogen-activated protein kinase termed salicylic acid-induced protein kinase (SIPK) in tobacco suspension cells. Here, we show that infiltration of these agents into tobacco leaves also activates SIPK. Of particular interest, infiltration of water alone activated a kinase of the same size, possibly because of wounding and/or osmotic stresses. The kinetics of kinase activation, however, differ for these different treatments. Various mechanical stresses, including cutting and wounding by abrasion, also activated a 48-kDa kinase. By using an immune-complex kinase assay with antibodies specific for SIPK or wounding-induced protein kinase, we demonstrate that this wounding-activated 48-kDa kinase is SIPK, rather than wounding-induced protein kinase, as reported [Seo, S., Okamoto, M., Seto, H., Ishizuka, K., Sano, H. & Ohashi, Y. (1995) Science 270, 1988-1992]. Activation of SIPK after wounding was associated with tyrosine phosphorylation but not with increases in SIPK mRNA or protein levels. Thus, the same mitogen-activated protein kinase, SIPK, appears to facilitate signaling for two distinct pathways that lead to disease resistance responses and wounding responses.
Figures




Similar articles
-
Activation of salicylic acid-induced protein kinase, a mitogen-activated protein kinase, induces multiple defense responses in tobacco.Plant Cell. 2001 Aug;13(8):1877-89. doi: 10.1105/tpc.010044. Plant Cell. 2001. PMID: 11487699 Free PMC article.
-
Molecular cloning and characterization of a tobacco MAP kinase kinase that interacts with SIPK.Mol Plant Microbe Interact. 2000 Jan;13(1):118-24. doi: 10.1094/MPMI.2000.13.1.118. Mol Plant Microbe Interact. 2000. PMID: 10656593
-
The mitogen-activated protein kinases WIPK and SIPK regulate the levels of jasmonic and salicylic acids in wounded tobacco plants.Plant J. 2007 Mar;49(5):899-909. doi: 10.1111/j.1365-313X.2006.03003.x. Epub 2007 Jan 23. Plant J. 2007. PMID: 17253983
-
Pathogen-induced MAP kinases in tobacco.Results Probl Cell Differ. 2000;27:65-84. doi: 10.1007/978-3-540-49166-8_6. Results Probl Cell Differ. 2000. PMID: 10533199 Review.
-
[Regulation role of reactive oxygen species and mitogen-activated protein kinases in plant stress signaling].Zhi Wu Sheng Li Yu Fen Zi Sheng Wu Xue Xue Bao. 2005 Feb;31(1):1-10. Zhi Wu Sheng Li Yu Fen Zi Sheng Wu Xue Xue Bao. 2005. PMID: 15692172 Review. Chinese.
Cited by
-
Involvement of the mitogen-activated protein kinase SIMK in regulation of root hair tip growth.EMBO J. 2002 Jul 1;21(13):3296-306. doi: 10.1093/emboj/cdf349. EMBO J. 2002. PMID: 12093731 Free PMC article.
-
Osmotic stress induces rapid activation of a salicylic acid-induced protein kinase and a homolog of protein kinase ASK1 in tobacco cells.Plant Cell. 2000 Jan;12(1):165-78. Plant Cell. 2000. PMID: 10634915 Free PMC article.
-
A harpin binding site in tobacco plasma membranes mediates activation of the pathogenesis-related gene HIN1 independent of extracellular calcium but dependent on mitogen-activated protein kinase activity.Plant Cell. 2001 May;13(5):1079-93. doi: 10.1105/tpc.13.5.1079. Plant Cell. 2001. PMID: 11340183 Free PMC article.
-
Temporal and tissue-specific expression of the tobacco ntf4 MAP kinase.Plant Mol Biol. 2001 Apr;45(6):679-89. doi: 10.1023/a:1010645431133. Plant Mol Biol. 2001. PMID: 11430430
-
Recent advances in plant early signaling in response to herbivory.Int J Mol Sci. 2011;12(6):3723-39. doi: 10.3390/ijms12063723. Epub 2011 Jun 7. Int J Mol Sci. 2011. PMID: 21747702 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

