Determinations of levels of human immunodeficiency virus type 1 RNA in plasma: reassessment of parameters affecting assay outcome. TUBE Meeting Workshop Attendees. Technology Utilization for HIV-1 Blood Evaluation and Standardization in Pediatrics
- PMID: 9620364
- PMCID: PMC104860
- DOI: 10.1128/JCM.36.6.1471-1479.1998
Determinations of levels of human immunodeficiency virus type 1 RNA in plasma: reassessment of parameters affecting assay outcome. TUBE Meeting Workshop Attendees. Technology Utilization for HIV-1 Blood Evaluation and Standardization in Pediatrics
Figures
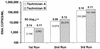
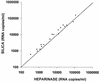

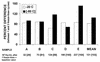
References
-
- Brambilla D, Leung S, Lew J, Todd J, Herman S, Cronin M, Shapiro D E, Bremer J, Hanson C, Hillyer G, McSherry G, Sperling R, Coombs R W, Reichelderer P S. Absolute copy number and relative change in plasma HIV-1 RNA determination: effects of an external standard on kit comparison. J Clin Microbiol. 1998;36:311–314. - PMC - PubMed
-
- Burchett S K, Kornegay J R, Pitt J, Landesman S H, Rosenblatt H M, Hillyer G V, Garcia P M, Kalish L A, Burns D N, Lew J F for the Women and Infants Transmission Study Group. Proceedings of the Third Conference of Retroviruses and Opportunistic Infections. 1996. Assessment of maternal plasma HIV viral load as a correlate of vertical transmission, abstr. LB3; p. 161.
-
- Burns D N, Landesman S, Wright D J, Waters D, Mitchell R M, Rubinstein A, Willoughby A, Goedert J J. Influence of other maternal variables on the relationship between maternal virus load and mother-to-infant transmission of human immunodeficiency virus type 1. J Infect Dis. 1997;175:1206–1210. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

