Notochord repression of endodermal Sonic hedgehog permits pancreas development
- PMID: 9620856
- PMCID: PMC316875
- DOI: 10.1101/gad.12.11.1705
Notochord repression of endodermal Sonic hedgehog permits pancreas development
Abstract
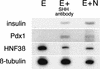
Notochord signals to the endoderm are required for development of the chick dorsal pancreas. Sonic hedgehog (SHH) is normally absent from pancreatic endoderm, and we provide evidence that notochord, in contrast to its effects on adjacent neuroectoderm where SHH expression is induced, represses SHH expression in adjacent nascent pancreatic endoderm. We identify activin-betaB and FGF2 as notochord factors that can repress endodermal SHH and thereby permit expression of pancreas genes including Pdx1 and insulin. Endoderm treatment with antibodies that block hedgehog activity also results in pancreatic gene expression. Prevention of SHH expression in prepancreatic dorsal endoderm by intercellular signals, like activin and FGF, may be critical for permitting early steps of chick pancreatic development.
Figures





References
-
- Ahlgren U, Jonsson J, Edlund H. The morphogenesis of the pancreatic mesenchyme is uncoupled from that of the pancreatic epithelium in IPF1/PDX1-deficient mice. Development. 1996;122:1409–1416. - PubMed
-
- Ahlgren U, Pfaff SL, Jessell TM, Edlund T, Edlund H. Independent requirement for ISL1 in formation of pancreatic mesenchyme and islet cells. Nature. 1997;385:257–260. - PubMed
-
- Apelqvist Å, Ahlgren U, Edlund H. Sonic hedgehog directs specialised mesoderm differentiation in the intestine and pancreas. Curr Biol. 1997;7:801–804. - PubMed
-
- Basler K, Edlund T, Jessell TM, Yamada T. Control of cell pattern in the neural tube: Regulation of cell differentiation by dorsalin-1, a novel TGFβ family member. Cell. 1993;73:687–702. - PubMed
-
- Beaupain D, Dieterlen-Lièvre F. Etude immunocytologique de la differenciation du pancreas endocrine chez l’embryon de poulet. II. Glucagon. Gen Comp Endocrinol. 1974;23:421–423. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
