Changes in ribosomal activity of Escherichia coli cells during prolonged culture in sea salts medium
- PMID: 9620960
- PMCID: PMC107811
- DOI: 10.1128/JB.180.12.3114-3119.1998
Changes in ribosomal activity of Escherichia coli cells during prolonged culture in sea salts medium
Abstract
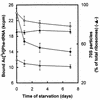
The activity of ribosomes from a clinical isolate of Escherichia coli, exposed to starvation for 7 days in sea salts medium, was investigated by measuring the kinetic parameters of ribosomal peptidyltransferase, by using the puromycin reaction as a model reaction. No alterations in the extent of peptide bond formation were observed during starvation. In contrast, a 50% reduction in the kmax/Ks ratio could be seen after 24 h of starvation; an additional 6 days of starvation resulted in a progressive but less abrupt decline in the kmax/Ks value. (kmax is the apparent catalytic rate constant of peptidyl transferase, and Ks is the dissociation constant of the encounter complex between acetyl (Ac)[3H]Phe-tRNA-poly(U)-ribosome and puromycin.) Although the distribution of ribosomal particles remained constant, a substantial decrease in the number of ribosomes per starved cell and a clear decline in the ability of ribosomes to bind AcPhe-tRNA were observed, particularly during the first day of starvation. Further analysis indicated that rRNA in general, but especially 23S rRNA, was rapidly degraded during the starvation period. In addition, the L12/L7 molar ratio decreased from 1.5 to 1 during the initial phase of starvation (up to 24 h) but remained constant during the subsequent starvation period. Ribosomes isolated from 24-h-starved cells, when artificially depleted of L7/L12 protein and reconstituted with L7/L12 protein from mid-logarithmic-phase cells, regenerated an L12/L7 molar ratio of 1.5 and restored the peptidyltransferase activity to a substantial level. An analogous effect of reconstitution on the efficiency of ribosomes in binding AcPhe-tRNA was evident not only during the initial phase but throughout the starvation period.
Figures



Similar articles
-
Growth phase and growth rate dependence of ribosomal peptidyltransferase activity status in Escherichia coli.Biochimie. 1995;77(12):963-71. doi: 10.1016/0300-9084(95)80009-3. Biochimie. 1995. PMID: 8834779
-
Ribosome-catalyzed peptide-bond formation with an A-site substrate covalently linked to 23S ribosomal RNA.Science. 1998 Apr 10;280(5361):286-9. doi: 10.1126/science.280.5361.286. Science. 1998. PMID: 9535658
-
[Puromycin interacts with the donor (P) site of Escherichia coli ribosomes].Mol Biol (Mosk). 1984 Sep-Oct;18(5):1301-5. Mol Biol (Mosk). 1984. PMID: 6390175 Russian.
-
23S rRNA nucleotides in the peptidyl transferase center are essential for tryptophanase operon induction.J Bacteriol. 2009 Jun;191(11):3445-50. doi: 10.1128/JB.00096-09. Epub 2009 Mar 27. J Bacteriol. 2009. PMID: 19329641 Free PMC article.
-
Peptidyl transferase and beyond.Biochem Cell Biol. 1995 Nov-Dec;73(11-12):1041-7. doi: 10.1139/o95-111. Biochem Cell Biol. 1995. PMID: 8722019 Review.
Cited by
-
Seasonal and spatial variability in Lake Michigan sediment small-subunit rRNA concentrations.Appl Environ Microbiol. 2001 Sep;67(9):3908-22. doi: 10.1128/AEM.67.9.3908-3922.2001. Appl Environ Microbiol. 2001. PMID: 11525985 Free PMC article.
-
Prokaryotic community analysis with CARD-FISH in comparison with FISH in ultra-oligotrophic ground- and drinking water.J Appl Microbiol. 2007 Oct;103(4):871-81. doi: 10.1111/j.1365-2672.2007.03319.x. J Appl Microbiol. 2007. PMID: 17897189 Free PMC article.
-
Inhibition of bacterial ribosome assembly: a suitable drug target?Microbiol Mol Biol Rev. 2009 Mar;73(1):22-35. doi: 10.1128/MMBR.00030-08. Microbiol Mol Biol Rev. 2009. PMID: 19258531 Free PMC article. Review.
References
-
- Albertson N H, Nystrom T. Effects of starvation for exogenous carbon on functional mRNA stability and rate of peptide chain elongation in Escherichia coli. FEMS Microbiol Lett. 1994;117:181–188. - PubMed
-
- Andrieux E, Cozzone A J. Conformational changes in bacterial polysomes induced by amino acid starvation. Int J Biochem. 1984;16:113–116. - PubMed
-
- Barritault D, Expert-Bezancon A, Guerin M F, Hayes D. The use of acetone precipitation in the isolation of ribosomal proteins. Eur J Biochem. 1976;63:131–135. - PubMed
-
- Daniel W W. Biostatistics: foundation for analysis in the health sciences. New York, N.Y: Wiley; 1978. pp. 284–303.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

