Archetype JC virus efficiently replicates in COS-7 cells, simian cells constitutively expressing simian virus 40 T antigen
- PMID: 9620986
- PMCID: PMC110153
- DOI: 10.1128/JVI.72.7.5335-5342.1998
Archetype JC virus efficiently replicates in COS-7 cells, simian cells constitutively expressing simian virus 40 T antigen
Abstract
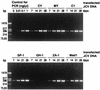
JC polyomavirus (JCV), the causative agent of progressive multifocal leukoencephalopathy (PML), is ubiquitous in humans, infecting children asymptomatically and then persisting in the kidney. Renal JCV is not latent but replicates to excrete progeny in the urine. The renal-urinary JCV DNAs carry the archetype regulatory region that generates various rearranged regulatory regions occurring in JCVs derived from the brains of PML patients. Tissue cultures that support the efficient growth of archetype JCV have not been reported. We studied whether archetype JCV could replicate in COS-7 cells, simian cells transformed with an origin-defective mutant of simian virus 40 (SV40). Efficient JCV replication, as detected by a hemagglutination assay, was observed in cultures transfected with five of the six archetype DNAs. The progeny JCVs could be passaged to fresh COS-7 cells. However, when the parental cells of COS-7 not expressing T antigen were transfected with archetype JCV DNAs, no viral replication was detected, indicating that SV40 T antigen is essential for the growth of JCV in COS-7 cells. The archetype regulatory region was conserved during viral growth in COS-7 cells, although a small proportion of JCV DNAs underwent rearrangements outside the regulatory region. We then attempted to recover archetype JCV from urine by viral culture in COS-7 cells. Efficient JCV production was observed in COS-7 cells infected with five of the six JCV-positive urine samples examined. Thus, COS-7 cells should be of use not only for the production of archetype JCV on a large scale but also for the isolation of archetype JCV from urine.
Figures

References
-
- Agostini H T, Brubaker G R, Shao J, Ryschkewitsch C F, Blattner W A, Stoner G L. BK virus and a new type of JC virus excreted by HIV-1-positive patients in rural Tanzania. Arch Virol. 1995;140:1919–1934. - PubMed
-
- Agostini H T, Ryschkewitsch C F, Brubaker G R, Shano J, Stoner G L. Five complete genomes of JC virus type 3 from Africans and African Americans. Arch Virol. 1997;142:637–655. - PubMed
-
- Agostini H T, Ryschkewitsch C F, Singer E J, Stoner G L. JC virus regulatory region rearrangements and genotypes in progressive multifocal leukoencephalopathy: two independent aspects of virus variation. J Gen Virol. 1997;78:659–664. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

