Virion-targeted viral inactivation of human immunodeficiency virus type 1 by using Vpr fusion proteins
- PMID: 9620999
- PMCID: PMC110178
- DOI: 10.1128/JVI.72.7.5441-5448.1998
Virion-targeted viral inactivation of human immunodeficiency virus type 1 by using Vpr fusion proteins
Abstract
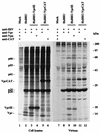
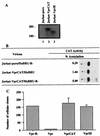
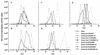
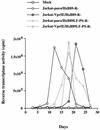
Inactivation of progeny virions with chimeric virion-associated proteins represents a novel therapeutic approach against human immunodeficiency virus (HIV) replication. The HIV type 1 (HIV-1) Vpr gene product, which is packaged into virions, is an attractive candidate for such a strategy. In this study, we developed Vpr-based fusion proteins that could be specifically targeted into mature HIV-1 virions to affect their structural organization and/or functional integrity. Two Vpr fusion proteins were constructed by fusing to the first 88 amino acids of HIV-1 Vpr the chloramphenicol acetyltransferase enzyme (VprCAT) or the last 18 C-terminal amino acids of the HIV-1 Vpu protein (VprIE). These Vpr fusion proteins were initially designed to quantify their efficiency of incorporation into HIV-1 virions when produced in cis from the provirus. Subsequently, CD4+ Jurkat T-cell lines constitutively expressing the VprCAT or the VprIE fusion protein were generated with retroviral vectors. Expression of the VprCAT or the VprIE fusion protein in CD4+ Jurkat T cells did not interfere with cellular viability or growth but conferred substantial resistance to HIV replication. The resistance to HIV replication was more pronounced in Jurkat-VprIE cells than in Jurkat-VprCAT cells. Moreover, the antiviral effect mediated by VprIE was dependent on an intact p6(gag) domain, indicating that the impairment of HIV-1 replication required the specific incorporation of Vpr fusion protein into virions. Gene expression, assembly, or release was not affected upon expression of these Vpr fusion proteins. Indeed, the VprIE and VprCAT fusion proteins were shown to affect the infectivity of progeny virus, since HIV virions containing the VprCAT or the VprIE fusion proteins were, respectively, 2 to 3 times and 10 to 30 times less infectious than the wild-type virus. Overall, this study demonstrated the successful transfer of resistance to HIV replication in tissue cultures by use of Vpr-based antiviral genes.
Figures
Similar articles
-
Inhibition of HIV-1 replication and infectivity by expression of a fusion protein, VPR-anti-integrase single-chain variable fragment (SFv): intravirion molecular therapies.J Hum Virol. 2000 Jan-Feb;3(1):6-15. J Hum Virol. 2000. PMID: 10774802
-
HIV-1 Vpr-chloramphenicol acetyltransferase fusion proteins: sequence requirement for virion incorporation and analysis of antiviral effect.Gene Ther. 1999 Sep;6(9):1590-9. doi: 10.1038/sj.gt.3300988. Gene Ther. 1999. PMID: 10490769
-
Incorporation of functional human immunodeficiency virus type 1 integrase into virions independent of the Gag-Pol precursor protein.J Virol. 1997 Oct;71(10):7704-10. doi: 10.1128/JVI.71.10.7704-7710.1997. J Virol. 1997. PMID: 9311854 Free PMC article.
-
Partner molecules of accessory protein Vpr of the human immunodeficiency virus type 1.DNA Cell Biol. 2004 Apr;23(4):193-205. doi: 10.1089/104454904773819789. DNA Cell Biol. 2004. PMID: 15142377 Review.
-
Vpr and Its Cellular Interaction Partners: R We There Yet?Cells. 2019 Oct 24;8(11):1310. doi: 10.3390/cells8111310. Cells. 2019. PMID: 31652959 Free PMC article. Review.
Cited by
-
Targeting the Virus Capsid as a Tool to Fight RNA Viruses.Viruses. 2022 Jan 18;14(2):174. doi: 10.3390/v14020174. Viruses. 2022. PMID: 35215767 Free PMC article. Review.
-
Gene therapy for HIV infections: Intracellular immunization.Can J Infect Dis. 1999 Jul;10(4):307-12. doi: 10.1155/1999/914379. Can J Infect Dis. 1999. PMID: 22346390 Free PMC article.
-
Vpr14-88-Apobec3G fusion protein is efficiently incorporated into Vif-positive HIV-1 particles and inhibits viral infection.PLoS One. 2008 Apr 16;3(4):e1995. doi: 10.1371/journal.pone.0001995. PLoS One. 2008. PMID: 18414671 Free PMC article.
-
Capsid-Targeted Viral Inactivation: A Novel Tactic for Inhibiting Replication in Viral Infections.Viruses. 2016 Sep 21;8(9):258. doi: 10.3390/v8090258. Viruses. 2016. PMID: 27657114 Free PMC article. Review.
-
Design of a trans protease lentiviral packaging system that produces high titer virus.Retrovirology. 2007 Dec 28;4:96. doi: 10.1186/1742-4690-4-96. Retrovirology. 2007. PMID: 18163907 Free PMC article.
References
-
- Baltimore D. Intracellular immunization. Nature (London) 1988;335:395–396. - PubMed
-
- Boeke J D, Hahn B. Destroying retroviruses from within. Trends Microbiol. 1996;4:421–426. - PubMed
-
- Bonyhadi M L, Moss K, Voytovich A, Auten J, Kalfoglou C, Plavec I, Forestell S, Su L, Böhnlein E, Kaneshima H. RevM10-expressing T cells derived in vivo from transduced human hematopoietic stem progenitor cells inhibit human immunodeficiency virus replication. J Virol. 1997;71:4707–4716. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

