Intracellular signaling by the chemokine receptor US28 during human cytomegalovirus infection
- PMID: 9621010
- PMCID: PMC110199
- DOI: 10.1128/JVI.72.7.5535-5544.1998
Intracellular signaling by the chemokine receptor US28 during human cytomegalovirus infection
Abstract
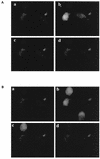
In patients with impaired cell-mediated immune responses (e.g., lung transplant recipients and AIDS patients), cytomegalovirus (CMV) infection causes severe disease such as pneumonitis. However, although immunocompetency in the host can protect from CMV disease, the virus persists by evading the host immune defenses. A model of CMV infection of the endothelium has been developed in which inflammatory stimuli, such as the CC chemokine RANTES, bind to the endothelial cell surface, stimulating calcium flux during late times of CMV infection. At 96 h postinfection, CMV-infected cells express mRNA of the CMV-encoded CC chemokine receptor US28 but do not express mRNA of other CC chemokine receptors that bind RANTES (CCR1, CCR4, CCR5). Cloning and stable expression of the receptor CMV US28 in human kidney epithelial cells (293 cells) with and without the heterotrimeric G protein alpha16 indicated that CMV US28 couples to both Galphai and Galpha16 proteins to activate calcium flux in response to the chemokines RANTES and MCP-3. Furthermore, cells that coexpress US28 and Galpha16 responded to RANTES stimulation with activation of extracellular signal-regulated kinase, which could be attributed, in part, to specific Galpha16 coupling. Thus, through expression of the CC chemokine receptor US28, CMV may utilize resident G proteins of the infected cell to manipulate cellular responses stimulated by chemokines.
Figures


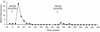






References
-
- AbuBaker S, Boldogh I, Albrecht T. HCMV, stimulation of [3H] release from [3H]-arachidonic acid prelabelled cells. Arch Virol. 1990;113:255–266. - PubMed
-
- Albrecht T, Boldogh I, Fons M. Receptor-initiated activation of cells and their oncogenes by herpes-family viruses. J Invest Dermatol. 1992;98:29S–35S. - PubMed
-
- Albrecht T, Boldogh I, Fons M, AbuBakar S, Deng C. Cell activation signals and the pathogenesis of HCMV. Intervirology. 1990;31:68–75. - PubMed
-
- Albrecht T, Boldogh I, Fons M, Lee C, AbuBakar S, Russell J, Au W. Cell-activation responses to CMV infection. Relationship to phasing of CMV replication and to the induction of cellular damage. Subcell Biochem. 1989;15:157–202. - PubMed
-
- Avdi N, Winston B W, Russel M, Young S K, Johnson G L, Worthen G S. Activation of MEKK by FMLP in human neutrophils: mapping pathways for MAP kinase activation. J Biol Chem. 1996;271:33598–33606. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

