Persistent activation of RelA by respiratory syncytial virus involves protein kinase C, underphosphorylated IkappaBbeta, and sequestration of protein phosphatase 2A by the viral phosphoprotein
- PMID: 9621019
- PMCID: PMC110221
- DOI: 10.1128/JVI.72.7.5610-5618.1998
Persistent activation of RelA by respiratory syncytial virus involves protein kinase C, underphosphorylated IkappaBbeta, and sequestration of protein phosphatase 2A by the viral phosphoprotein
Abstract
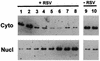
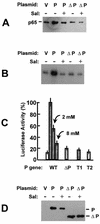
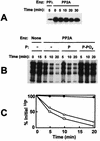
Respiratory syncytial virus (RSV) activated the RelA (p65) subunit of nuclear factor kappa B (NF-kappaB) over many hours postinfection. The initial activation coincided with phosphorylation and degradation of IkappaBalpha, the cytoplasmic inhibitor of RelA. During persistent activation of NF-kappaB at later times in infection, syntheses of inhibitors IkappaBalpha as well as IkappaBbeta were restored. However, the resynthesized IkappaBbeta was in an underphosphorylated state, which apparently prevented inhibition of NF-kappaB. Use of specific inhibitors suggested that the pathway leading to the persistent-but not the initial-activation of NF-kappaB involved signaling through protein kinase C (PKC) and reactive oxygen intermediates of nonmitochondrial origin, whereas phospholipase C or D played little or no role. Thus, RSV infection led to the activation of NF-kappaB by a biphasic mechanism: a transient or early activation involving phosphorylation of the inhibitor IkappaB polypeptides, and a persistent or long-term activation requiring PKC and the generation of hypophosphorylated IkappaBbeta. At least a part of the activation was through a novel mechanism in which the viral phosphoprotein P associated with but was not dephosphorylated by protein phosphatase 2A and thus sequestered and inhibited the latter. We postulate that this led to a net increase in the phosphorylation state of signaling proteins that are responsible for RelA activation.
Figures




References
-
- Amtmann E. The antiviral, antitumoural xanthate D609 is a competitive inhibitor of phosphatidylcholine-specific phospholipase C. Drugs Exp Clin Res. 1996;22:287–294. - PubMed
-
- Anderson L J, Heilman C A. Protective and disease-enhancing immune responses to respiratory syncytial virus. J Infect Dis. 1995;171:1–7. - PubMed
-
- Ansai T, Dupuy L C, Barik S. Interactions between a minimal protein serine/threonine phosphatase and its phosphopeptide substrate sequence. J Biol Chem. 1996;271:24401–24407. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

