Determination of the nucleotide sequence of Bombyx mori cytoplasmic polyhedrosis virus segment 9 and its expression in BmN4 cells
- PMID: 9621035
- PMCID: PMC110377
- DOI: 10.1128/JVI.72.7.5762-5768.1998
Determination of the nucleotide sequence of Bombyx mori cytoplasmic polyhedrosis virus segment 9 and its expression in BmN4 cells
Abstract
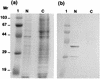
Cloning and sequencing of segment 9 of Bombyx mori cytoplasmic polyhedrosis virus (BmCPV) strains H and I were performed. The segment consisted of 1,186 bp harboring 5' and 3' noncoding regions and an open reading frame from positions 75 to 1037, encoding a protein with 320 amino acids, termed NS5. Comparison of the nucleotide sequences of NS5 for the two strains indicated 37 point differences resulting in only six amino acid replacements. Homology search showed that NS5 has localized similarities to human poliovirus RNA-dependent RNA polymerase and human rotavirus NS26. By Western blot analysis, NS5 was found in BmCPV-infected midgut cells, but not in polyhedra or virus virions, and was mainly detectable in the nucleus in BmCPV-infected BmN4 cells. Immunoblot analysis with anti-NS5 and antipolyhedrin antibodies displayed marked differences in the period of expression of NS5 and polyhedrin: the polyhedrin molecule was first detected 2 or 3 days after infection with BmCPV, whereas the expression of NS5 was initiated within a few hours. In addition, the level of polyhedrin increased as the infection developed, whereas the amount of NS5 remained essentially constant. When segment 9 was expressed with a baculovirus expression system, the resulting NS5 protein possessed the ability to bind to the double-stranded RNA genome. These results suggest that NS5 is expressed in early stages of infection and contributes to regulation of genomic RNA function.
Figures







References
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Research Materials

