DNA packaging mutant: repression of the vaccinia virus A32 gene results in noninfectious, DNA-deficient, spherical, enveloped particles
- PMID: 9621036
- PMCID: PMC110378
- DOI: 10.1128/JVI.72.7.5769-5780.1998
DNA packaging mutant: repression of the vaccinia virus A32 gene results in noninfectious, DNA-deficient, spherical, enveloped particles
Abstract
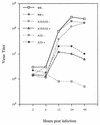
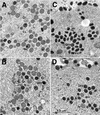
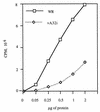
The vaccinia virus A32 open reading frame was predicted to encode a protein with a nucleoside triphosphate-binding motif and a mass of 34 kDa. To investigate the role of this protein, we constructed a mutant in which the original A32 gene was replaced by an inducible copy. The recombinant virus, vA32i, has a conditional lethal phenotype: infectious virus formation was dependent on isopropyl-beta-D-thiogalactopyranoside (IPTG). Under nonpermissive conditions, the mutant synthesized early- and late-stage viral proteins, as well as viral DNA that was processed into unit-length genomes. Electron microscopy of cells infected in the absence of IPTG revealed normal-appearing crescents and immature virus particles but very few with nucleoids. Instead of brick-shaped mature particles with defined core structures, there were numerous electron-dense, spherical particles. Some of these spherical particles were wrapped with cisternal membranes, analogous to intracellular and extracellular enveloped virions. Mutant viral particles, purified by sucrose density gradient centrifugation, had low infectivity and transcriptional activity, and the majority were spherical and lacked DNA. Nevertheless, the particle preparation contained representative membrane proteins, cleaved and uncleaved core proteins, the viral RNA polymerase, the early transcription factor and several enzymes, suggesting that incorporation of these components is not strictly coupled to DNA packaging.
Figures







References
-
- Baroudy B M, Venkatesan S, Moss B. Incompletely base-paired flip-flop terminal loops link the two DNA strands of the vaccinia virus genome into one uninterrupted polynucleotide chain. Cell. 1982;28:315–324. - PubMed
-
- Baroudy B M, Venkatesan S, Moss B. Structure and replication of vaccinia virus telomeres. Cold Spring Harbor Symp Quant Biol. 1982;47:723–729. - PubMed
-
- Broyles S S, Yuen L, Shuman S, Moss B. Purification of a factor required for transcription of vaccinia virus early genes. J Biol Chem. 1988;263:10754–10760. - PubMed
-
- Carroll M, Moss B. Host range and cytopathogenicity of the highly attenuated MVA strain of vaccinia virus: propagation and generation of recombinant viruses in a nonhuman mammalian cell line. Virology. 1997;238:198–211. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

