Role of the SH3-ligand domain of simian immunodeficiency virus Nef in interaction with Nef-associated kinase and simian AIDS in rhesus macaques
- PMID: 9621042
- PMCID: PMC110384
- DOI: 10.1128/JVI.72.7.5820-5830.1998
Role of the SH3-ligand domain of simian immunodeficiency virus Nef in interaction with Nef-associated kinase and simian AIDS in rhesus macaques
Abstract
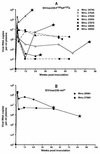
The nef gene of the human and simian immunodeficiency viruses (HIV and SIV) is dispensable for viral replication in T-cell lines; however, it is essential for high virus loads and progression to simian AIDS (SAIDS) in SIV-infected adult rhesus macaques. Nef proteins from HIV type 1 (HIV-1), HIV-2, and SIV contain a proline-Xaa-Xaa-proline (PxxP) motif. The region of Nef with this motif is similar to the Src homology region 3 (SH3) ligand domain found in many cell signaling proteins. In virus-infected lymphoid cells, Nef interacts with a cellular serine/threonine kinase, designated Nef-associated kinase (NAK). In this study, analysis of viral clones containing point mutations in the nef gene of the pathogenic clone SIVmac239 revealed that several strictly conserved residues in the PxxP region were essential for Nef-NAK interaction. The results of this analysis of Nef mutations in in vitro kinase assays indicated that the PxxP region in SIV Nef was strikingly similar to the consensus sequence for SH3 ligand domains possessing the minus orientation. To test the significance of the PxxP motif of Nef for viral pathogenesis, each proline was mutated to an alanine to produce the viral clone SIVmac239-P104A/P107A. This clone, expressing Nef that does not associate with NAK, was inoculated into seven juvenile rhesus macaques. In vitro kinase assays were performed on virus recovered from each animal; the ability of Nef to associate with NAK was restored in five of these animals as early as 8 weeks after infection. Analysis of nef genes from these viruses revealed patterns of genotypic reversion in the mutated PxxP motif. These revertant genotypes, which included a second-site suppressor mutation, restored the ability of Nef to interact with NAK. Additionally, the proportion of revertant viruses increased progressively during the course of infection in these animals, and two of these animals developed fatal SAIDS. Taken together, these results demonstrated that in vivo selection for the ability of SIV Nef to associate with NAK was correlated with the induction of SAIDS. Accordingly, these studies implicate a role for the conserved SH3 ligand domain for Nef function in virally induced immunodeficiency.
Figures


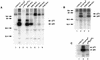
Similar articles
-
Simian immunodeficiency virus containing mutations in N-terminal tyrosine residues and in the PxxP motif in Nef replicates efficiently in rhesus macaques.J Virol. 2000 May;74(9):4155-64. doi: 10.1128/jvi.74.9.4155-4164.2000. J Virol. 2000. PMID: 10756028 Free PMC article.
-
SIV/HIV Nef recombinant virus (SHIVnef) produces simian AIDS in rhesus macaques.Virology. 1999 Dec 20;265(2):235-51. doi: 10.1006/viro.1999.0051. Virology. 1999. PMID: 10600596
-
Activation of PAK by HIV and SIV Nef: importance for AIDS in rhesus macaques.Curr Biol. 1996 Nov 1;6(11):1519-27. doi: 10.1016/s0960-9822(96)00757-9. Curr Biol. 1996. PMID: 8939608
-
Interactions of the HIV/SIV pathogenicity factor Nef with SH3 domain-containing host cell proteins.Curr HIV Res. 2011 Oct;9(7):531-42. doi: 10.2174/157016211798842107. Curr HIV Res. 2011. PMID: 22103837 Review.
-
Nef and the Nef-associated kinase.Res Virol. 1997 Jan-Feb;148(1):47-52. doi: 10.1016/s0923-2516(97)81913-9. Res Virol. 1997. PMID: 9017834 Review. No abstract available.
Cited by
-
HIV-1 Balances the Fitness Costs and Benefits of Disrupting the Host Cell Actin Cytoskeleton Early after Mucosal Transmission.Cell Host Microbe. 2019 Jan 9;25(1):73-86.e5. doi: 10.1016/j.chom.2018.12.008. Cell Host Microbe. 2019. PMID: 30629922 Free PMC article.
-
p21-activated kinase 1 plays a critical role in cellular activation by Nef.Mol Cell Biol. 2000 Apr;20(7):2619-27. doi: 10.1128/MCB.20.7.2619-2627.2000. Mol Cell Biol. 2000. PMID: 10713183 Free PMC article.
-
Human immunodeficiency virus type 1 inhibits DNA damage-triggered apoptosis by a Nef-independent mechanism.J Virol. 2005 May;79(9):5489-98. doi: 10.1128/JVI.79.9.5489-5498.2005. J Virol. 2005. PMID: 15827163 Free PMC article.
-
Association of Nef with p21-activated kinase 2 is dispensable for efficient human immunodeficiency virus type 1 replication and cytopathicity in ex vivo-infected human lymphoid tissue.J Virol. 2007 Dec;81(23):13005-14. doi: 10.1128/JVI.01436-07. Epub 2007 Sep 19. J Virol. 2007. PMID: 17881449 Free PMC article.
-
The soluble ectodomain of herpes simplex virus gD contains a membrane-proximal pro-fusion domain and suffices to mediate virus entry.Proc Natl Acad Sci U S A. 2004 May 11;101(19):7445-50. doi: 10.1073/pnas.0401883101. Epub 2004 May 3. Proc Natl Acad Sci U S A. 2004. PMID: 15123804 Free PMC article.
References
-
- Allan J S. Human immunodeficiency virus-related infections in animal models. In: Devita V T, Hellman S, Rosenberg S A, editors. AIDS—etiology, diagnosis, treatment and prevention. Philadelphia, Pa: Lippincott-Raven; 1997. pp. 15–27.
-
- Baba T W, Jeong Y S, Pennick D, Bronson R, Greene M F, Ruprecht R M. Pathogenicity of live, attenuated SIV after mucosal infection of neonatal macaques. Science. 1995;267:1820–1825. - PubMed
-
- Banapour B, Marthas M L, Munn R J, Luciw P A. In vitro macrophage tropism of pathogenic and nonpathogenic molecular clones of simian immunodeficiency virus (SIVmac) Virology. 1991;183:12–19. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

