Retinoid-induced repression of human immunodeficiency virus type 1 core promoter activity inhibits virus replication
- PMID: 9621047
- PMCID: PMC110389
- DOI: 10.1128/JVI.72.7.5862-5869.1998
Retinoid-induced repression of human immunodeficiency virus type 1 core promoter activity inhibits virus replication
Abstract
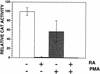
The rates of mother-to-child transmission of human immunodeficiency virus type 1 (HIV-1), progression to AIDS following HIV-1 infection, and AIDS-associated mortality are all inversely correlated with serum vitamin A levels (R. D. Semba, W. T. Caiaffa, N. M. H. Graham, S. Cohn, and D. Vlahov, J. Infect. Dis. 171:1196-1202, 1995; R. D. Semba, N. M. H. Graham, W. T. Caiaffa, J. B. Margolik, L. Clement, and D. Vlahov, Arch. Intern. Med. 153:2149-2154, 1993; R. D. Semba, P. G. Miotti, J. D. Chiphangwi, A. J. Saah, J. K. Canner, G. A. Dallabetta, and D. R. Hoover, Lancet 343:1593-1596, 1994). Here we show that physiological concentrations of vitamin A, as retinol or as its metabolite, all-trans retinoic acid, repressed HIV-1Ba-L replication in monocyte-derived macrophages (MDMs). Repression required retinoid treatment of peripheral monocytes during their in vitro differentiation into MDMs. Retinoids had no repressive effect if they were added after virus infection. Retinol, as well as all-trans retinoic acid and 9-cis retinoic acid, also repressed HIV-1 long terminal repeat (LTR)-directed expression up to 200-fold in transfected THP-1 monocytes. Analysis of HIV-1 LTR deletion mutants demonstrated that retinoids were able to repress activation of HIV-1 expression by both NF-kappaB and Tat. A cis-acting sequence required for retinoid-mediated repression of HIV-1 transcription was localized between nucleotides -51 and +12 of the HIV-1 LTR within the core promoter. Protein-DNA cross-linking experiments identified four proteins specific to retinoid-treated cells that bound to the core promoter. We conclude that retinoids render macrophages resistant to virus replication by modulating the interaction of cellular transcription factors with the viral core promoter.
Figures
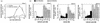
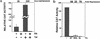




References
-
- Andersson S, Davis D N, Dahlback H, Jornvall H, Russell D W. Cloning, structure, and expression of the mitochondrial cytochrome P-450 sterol 26-hydroxylase, a bile acid biosynthetic enzyme. J Biol Chem. 1989;264:8222–8229. - PubMed
-
- Berkenstam A, Ruiz M M, Barettino D, Horikoshi M, Stunnenberg H G. Cooperativity in transactivation between retinoic acid receptor and TFIID requires an activity analogous to E1A. Cell. 1992;69:401–412. - PubMed
-
- Cho Y, Tighe A P, Talmage D A. Retinoic acid induced growth arrest of human breast carcinoma cells requires protein kinase C alpha expression and activity. J Cell Physiol. 1997;172:306–313. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

