Examination of the kinetics of herpes simplex virus glycoprotein D binding to the herpesvirus entry mediator, using surface plasmon resonance
- PMID: 9621056
- PMCID: PMC110398
- DOI: 10.1128/JVI.72.7.5937-5947.1998
Examination of the kinetics of herpes simplex virus glycoprotein D binding to the herpesvirus entry mediator, using surface plasmon resonance
Abstract
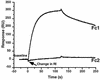
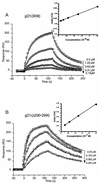
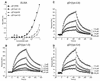
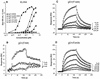
Previously, we showed that truncated soluble forms of herpes simplex virus (HSV) glycoprotein D (gDt) bound directly to a truncated soluble form of the herpesvirus entry mediator (HveAt, formerly HVEMt), a cellular receptor for HSV. The purpose of the present study was to determine the affinity of gDt for HveAt by surface plasmon resonance and to compare and contrast the kinetics of an expanded panel of gDt variants in binding to HveAt in an effort to better understand the mechanism of receptor binding and virus entry. Both HveAt and gDt are dimers in solution and interact with a 2:1 stoichiometry. With HveAt, gD1(306t) (from the KOS strain of HSV-1) had a dissociation constant (KD) of 3.2 x 10(-6) M and gD2(306t) had a KD of 1.5 x 10(-6) M. The interaction between gDt and HveAt fits a 1:1 Langmuir binding model, i.e., two dimers of HveAt may act as one binding unit to interact with one dimer of gDt as the second binding unit. A gD variant lacking all signals for N-linked oligosaccharides had an affinity for HveAt similar to that of gD1(306t). A variant lacking the bond from cysteine 1 to cysteine 5 had an affinity for HveAt that did not differ from that of the wild type. However, variants with double cysteine mutations that eliminated either of the other two disulfide bonds showed decreased affinity for HveAt. This result suggests that two of the three disulfide bonds of gD are important for receptor binding. Four nonfunctional gDt variants, each representing one functional domain of gD, were also studied. Mutations in functional regions I and II drastically decreased the affinity of gDt for HveAt. Surprisingly, a variant with an insertion in functional region III had a wild-type level of affinity for HveAt, suggesting that this domain may function in virus entry at a step other than receptor binding. A variant with a deletion in functional region IV [gD1(Delta290-299t)] exhibited a 100-fold enhancement in affinity for HveAt (KD = 3.3 x 10(-8) M) due mainly to a 40-fold increase in its kinetic on rate. This agrees with the results of other studies showing the enhanced ability of gD1(Delta290-299t) to block infection. Interestingly, all the variants with decreased affinities for HveAt exhibited decreased kinetic on rates but only minor changes in their kinetic off rates. The results suggest that once the complex between gDt and HveAt forms, its stability is unaffected by a variety of changes in gD.
Figures

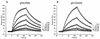
Similar articles
-
Functional region IV of glycoprotein D from herpes simplex virus modulates glycoprotein binding to the herpesvirus entry mediator.J Virol. 1998 Sep;72(9):7091-8. doi: 10.1128/JVI.72.9.7091-7098.1998. J Virol. 1998. PMID: 9696802 Free PMC article.
-
Glycoprotein D of herpes simplex virus (HSV) binds directly to HVEM, a member of the tumor necrosis factor receptor superfamily and a mediator of HSV entry.J Virol. 1997 Aug;71(8):6083-93. doi: 10.1128/JVI.71.8.6083-6093.1997. J Virol. 1997. PMID: 9223502 Free PMC article.
-
Monoclonal antibodies to distinct sites on herpes simplex virus (HSV) glycoprotein D block HSV binding to HVEM.J Virol. 1998 May;72(5):3595-601. doi: 10.1128/JVI.72.5.3595-3601.1998. J Virol. 1998. PMID: 9557640 Free PMC article.
-
The major neutralizing antigenic site on herpes simplex virus glycoprotein D overlaps a receptor-binding domain.J Virol. 1999 Dec;73(12):9879-90. doi: 10.1128/JVI.73.12.9879-9890.1999. J Virol. 1999. PMID: 10559300 Free PMC article.
-
The role of herpes simplex virus glycoproteins in the virus replication cycle.Acta Virol. 1998 Apr;42(2):103-18. Acta Virol. 1998. PMID: 9770079 Review.
Cited by
-
Immunisation Using Novel DNA Vaccine Encoding Virus Membrane Fusion Complex and Chemokine Genes Shows High Protection from HSV-2.Viruses. 2022 Oct 22;14(11):2317. doi: 10.3390/v14112317. Viruses. 2022. PMID: 36366414 Free PMC article.
-
Herpes virus fusion and entry: a story with many characters.Viruses. 2012 May;4(5):800-32. doi: 10.3390/v4050800. Epub 2012 May 10. Viruses. 2012. PMID: 22754650 Free PMC article. Review.
-
Separation of receptor-binding and profusogenic domains of glycoprotein D of herpes simplex virus 1 into distinct interacting proteins.Proc Natl Acad Sci U S A. 2007 Mar 6;104(10):4142-6. doi: 10.1073/pnas.0611565104. Epub 2007 Feb 27. Proc Natl Acad Sci U S A. 2007. PMID: 17360490 Free PMC article.
-
Mutations in herpes simplex virus glycoprotein D distinguish entry of free virus from cell-cell spread.J Virol. 2000 Dec;74(24):11437-46. doi: 10.1128/jvi.74.24.11437-11446.2000. J Virol. 2000. PMID: 11090139 Free PMC article.
-
Engineered disulfide bonds in herpes simplex virus type 1 gD separate receptor binding from fusion initiation and viral entry.J Virol. 2008 Jan;82(2):700-9. doi: 10.1128/JVI.02192-07. Epub 2007 Nov 21. J Virol. 2008. PMID: 18032483 Free PMC article.
References
-
- Baker S J, Reddy E P. Transducers of life and death: TNF receptor superfamily and associated proteins. Oncogene. 1996;12:1–9. - PubMed
-
- Biacore A B. BIAevaluation software handbook, version 3.0. Uppsala, Sweden: Biacore AB; 1997.
-
- Casasnovas J M, Springer T A. Kinetics and thermodynamics of virus binding to receptor. J Biol Chem. 1995;270:13216–13224. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

