Interaction of the human immunodeficiency virus type 1 Vpr protein with the nuclear pore complex
- PMID: 9621063
- PMCID: PMC110405
- DOI: 10.1128/JVI.72.7.6004-6013.1998
Interaction of the human immunodeficiency virus type 1 Vpr protein with the nuclear pore complex
Abstract
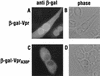
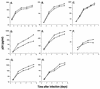
The Vpr protein of human immunodeficiency virus type 1 (HIV-1) performs a number of functions that are associated with the nucleus. Vpr enhances the nuclear import of postentry viral nucleoprotein complexes, arrests proliferating cells in the G2 phase of the cell cycle, and acts as a modest transcriptional activator. For this paper, we have investigated the nuclear import of Vpr. Although Vpr does not encode a sequence that is recognizable as a nuclear localization signal (NLS), Vpr functions as a transferable NLS both in somatic cells and in Xenopus laevis oocytes. In certain contexts, Vpr also mediates substantial accumulation at the nuclear envelope and, in particular, at nuclear pore complexes (NPCs). Consistent with this, Vpr is shown to interact specifically with nucleoporin phenylalanine-glycine (FG)-repeat regions. These findings not only demonstrate that Vpr harbors a bona fide NLS but also raise the possibility that one (or more) of Vpr's functions may take place at the NPC.
Figures




References
-
- Agostini I, Navarro J-M, Rey F, Bouhamdan M, Spire B, Vigne R, Sire J. The human immunodeficiency virus type 1 Vpr transactivator: cooperation with promoter-bound activator domains and binding to TFIIB. J Mol Biol. 1996;261:599–606. - PubMed
-
- Aitchison J D, Blobel G, Rout M P. Kap104p: a karyopherin involved in the nuclear transport of messenger RNA binding proteins. Science. 1996;274:624–627. - PubMed
-
- Ayyavoo V, Mahboubi A, Mahalingam S, Ramalingam R, Kudchodkar S, Williams W V, Green D R, Weiner D B. HIV-1 Vpr suppresses immune activation and apoptosis through regulation of nuclear factor κB. Nat Med. 1997;3:1117–1123. - PubMed
-
- Balliet J W, Kolson D L, Eiger G, Kim F M, McGann K A, Srinivasan A, Collman R. Distinct effects in primary macrophages and lymphocytes of the human immunodeficiency virus type 1 accessory genes vpr, vpu, and nef: mutational analysis of a primary HIV-1 isolate. Virology. 1994;200:623–631. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

