Particle polymorphism caused by deletion of a peptide molecular switch in a quasiequivalent icosahedral virus
- PMID: 9621065
- PMCID: PMC110407
- DOI: 10.1128/JVI.72.7.6024-6033.1998
Particle polymorphism caused by deletion of a peptide molecular switch in a quasiequivalent icosahedral virus
Abstract
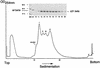
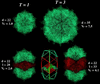
The capsid of flock house virus is composed of 180 copies of a single type of coat protein which forms a T=3 icosahedral shell. High-resolution structural analysis has shown that the protein subunits, although chemically identical, form different contacts across the twofold axes of the virus particle. Subunits that are related by icosahedral twofold symmetry form flat contacts, whereas subunits that are related by quasi-twofold symmetry form bent contacts. The flat contacts are due to the presence of ordered genomic RNA and an ordered peptide arm which is inserted in the groove between the subunits and prevents them from forming the dihedral angle observed at the bent quasi-twofold contacts. We hypothesized that by deleting the residues that constitute the ordered peptide arm, formation of flat contacts should be impossible and therefore result in assembly of particles with only bent contacts. Such particles would have T=1 symmetry. To test this hypothesis we generated two deletion mutants in which either 50 or 31 residues were eliminated from the N terminus of the coat protein. We found that in the absence of residues 1 to 50, assembly was completely inhibited, presumably because the mutation removed a cluster of positively charged amino acids required for neutralization of encapsidated RNA. When the deletion was restricted to residues 1 to 31, assembly occurred, but the products were highly heterogeneous. Small bacilliform-like structures and irregular structures as well as wild-type-like T=3 particles were detected. The anticipated T=1 particles, on the other hand, were not observed. We conclude that residues 20 to 30 are not critical for formation of flat protein contacts and formation of T=3 particles. However, the N terminus of the coat protein appears to play an essential role in regulating assembly such that only one product, T=3 particles, is synthesized.
Figures

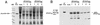



References
-
- Davis T R, Wickham T J, McKenna K A, Granados R R, Shuler M L, Wood H A. Comparative recombinant protein production of eight insect cell lines. In Vitro Cell Dev Biol Anim. 1993;29A:388–390. - PubMed
-
- Earnshaw W, King J. Structure of phage P22 coat protein aggregates formed in the absence of the scaffolding protein. J Mol Biol. 1978;126:721–747. - PubMed
-
- Erickson J W, Rossmann M G. Assembly and crystallization of a T = 1 icosahedral particle from trypsinized southern bean mosaic virus coat protein. Virology. 1982;116:128–136. - PubMed
-
- Fisher A J, Johnson J E. Ordered duplex RNA controls capsid architecture in an icosahedral animal virus. Nature. 1993;361:176–179. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

