Up to 100-fold increase of apparent gene expression in the presence of Epstein-Barr virus oriP sequences and EBNA1: implications of the nuclear import of plasmids
- PMID: 9621086
- PMCID: PMC110430
- DOI: 10.1128/JVI.72.7.6181-6185.1998
Up to 100-fold increase of apparent gene expression in the presence of Epstein-Barr virus oriP sequences and EBNA1: implications of the nuclear import of plasmids
Abstract
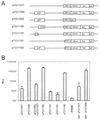
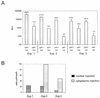
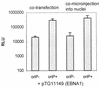
A 100-fold increase in luciferase activity was observed in 293 cells, stably expressing Epstein-Barr nuclear antigen 1 (EBNA1; 293-EBNA1 cells), that had been transiently transfected with plasmids carrying Epstein-Barr virus (EBV) oriP sequences. This increase was observed in comparison to reporter gene activity obtained after transfection with a plasmid carrying no oriP sequences. The luciferase gene on these plasmids was under the control of either the cytomegalovirus immediate-early 1 gene enhancer-promoter (CMV IE1) or the Rous sarcoma virus promoter. The increase of reporter gene activity was not due to plasmid replication, since a similar enhancement was observed in the presence of aphidicolin, an inhibitor of replicative DNA synthesis, or after deletion of the dyad symmetry (DS) element within oriP. Luciferase production was not increased in the presence of only the DS element. Microinjection of plasmids carrying the CMV IE1 promoter-driven luciferase gene with or without oriP sequences into the nuclei of 293-EBNA1 cells resulted in a 17-fold increase in luciferase activity. Cytoplasmic injection of these plasmids led to an enhancement of luciferase activity of up to 100-fold. This difference in the factor of activation after nuclear or cytoplasmic injection could be ascribed to increased transport of plasmids carrying oriP from the cytoplasm to the nucleus in the presence of EBNA1. These data suggest the possibility of substantially increasing the apparent expression of a gene under the control of a strong constitutive promoter in the presence of oriP sequences and EBNA1. This improvement in expression is due to intranuclear enhancement of gene expression. oriP-specific transport of plasmid DNA from the cytoplasm of 293-EBNA1 cells to the nucleus seems to contribute to the observed effect.
Figures
References
-
- Baer R, Bankier A T, Biggin M D, Deininger P L, Farrell P J, Gibson T J, Hatfull G, Hudson G S, Satchwell S C, Seguin C, Tuffnell P S, Barrell B G. DNA sequence and expression of the B95-8 Epstein-Barr virus genome. Nature. 1984;310:207–209. - PubMed
-
- Boshart M, Weber F, Jahn G, Dorsch-Haesler K, Fleckenstein B, Schaffner W. A very strong enhancer is located upstream of an immediate early gene of human cytomegalovirus. Cell. 1958;41:521–530. - PubMed
-
- Dean D. Import of plasmid DNA into the nucleus is sequence specific. Exp Cell Res. 1997;230:293–302. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

