The protein tyrosine kinase p56lck is required for triggering NF-kappaB activation upon interaction of human immunodeficiency virus type 1 envelope glycoprotein gp120 with cell surface CD4
- PMID: 9621091
- PMCID: PMC110439
- DOI: 10.1128/JVI.72.7.6207-6214.1998
The protein tyrosine kinase p56lck is required for triggering NF-kappaB activation upon interaction of human immunodeficiency virus type 1 envelope glycoprotein gp120 with cell surface CD4
Abstract
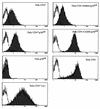
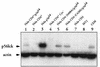
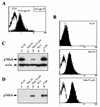
We have previously shown that NF-kappaB nuclear translocation can be observed upon human immunodeficiency virus type 1 (HIV-1) binding to cells expressing the wild-type CD4 molecule, but not in cells expressing a truncated form of CD4 that lacks the cytoplasmic domain (M. Benkirane, K.-T. Jeang, and C. Devaux, EMBO J. 13:5559-5569, 1994). This result indicated that the signaling cascade which controls HIV-1-induced NF-kappaB activation requires the integrity of the CD4 cytoplasmic tail and suggested the involvement of a second protein that binds to this portion of the molecule. Here we investigate the putative role of p56(lck) as a possible cellular intermediate in this signal transduction pathway. Using human cervical carcinoma HeLa cells stably expressing CD4, p56(lck), or both molecules, we provide direct evidence that expression of CD4 and p56(lck) is required for HIV-1-induced NF-kappaB translocation. Moreover, the fact that HIV-1 stimulation did not induce nuclear translocation of NF-kappaB in cells expressing a mutant form of CD4 at position 420 (C420A) and the wild-type p56(lck) indicates the requirement for a functional CD4-p56(lck) complex.
Figures



Similar articles
-
Transduction of activation signal that follows HIV-1 binding to CD4 and CD4 dimerization involves the immunoglobulin CDR3-like region in domain 1 of CD4.J Biol Chem. 1997 Aug 1;272(31):19441-50. doi: 10.1074/jbc.272.31.19441. J Biol Chem. 1997. PMID: 9235945
-
Repression of human immunodeficiency virus type 1 long terminal repeat-driven gene expression by binding of the virus to its primary cellular receptor, the CD4 molecule.J Virol. 1996 Jun;70(6):4009-16. doi: 10.1128/JVI.70.6.4009-4016.1996. J Virol. 1996. PMID: 8648738 Free PMC article.
-
The Ick protein tyrosine kinase is not involved in antibody-mediated CD4 (CDR3-loop) signal transduction that inhibits HIV-1 transcription.Eur J Immunol. 1998 May;28(5):1445-57. doi: 10.1002/(SICI)1521-4141(199805)28:05<1445::AID-IMMU1445>3.0.CO;2-P. Eur J Immunol. 1998. PMID: 9603449
-
Human immunodeficiency virus type 1-associated CD4 downmodulation.Adv Virus Res. 1994;44:203-66. doi: 10.1016/s0065-3527(08)60330-9. Adv Virus Res. 1994. PMID: 7817874 Free PMC article. Review.
-
Bioactive CD4 ligands as pre- and/or postbinding inhibitors of HIV-1.Adv Pharmacol. 2000;48:373-407. doi: 10.1016/s1054-3589(00)48012-9. Adv Pharmacol. 2000. PMID: 10987097 Review. No abstract available.
Cited by
-
SARS-CoV-2, ACE2, and Hydroxychloroquine: Cardiovascular Complications, Therapeutics, and Clinical Readouts in the Current Settings.Pathogens. 2020 Jul 7;9(7):546. doi: 10.3390/pathogens9070546. Pathogens. 2020. PMID: 32645974 Free PMC article. Review.
-
HIV: cell binding and entry.Cold Spring Harb Perspect Med. 2012 Aug 1;2(8):a006866. doi: 10.1101/cshperspect.a006866. Cold Spring Harb Perspect Med. 2012. PMID: 22908191 Free PMC article. Review.
-
Postentry restriction to human immunodeficiency virus-based vector transduction in human monocytes.J Virol. 2001 Jun;75(12):5448-56. doi: 10.1128/JVI.75.12.5448-5456.2001. J Virol. 2001. PMID: 11356951 Free PMC article.
-
New insights on the antiviral effects of chloroquine against coronavirus: what to expect for COVID-19?Int J Antimicrob Agents. 2020 May;55(5):105938. doi: 10.1016/j.ijantimicag.2020.105938. Epub 2020 Mar 12. Int J Antimicrob Agents. 2020. PMID: 32171740 Free PMC article.
-
HIV Envelope gp120 Alters T Cell Receptor Mobilization in the Immunological Synapse of Uninfected CD4 T Cells and Augments T Cell Activation.J Virol. 2016 Nov 14;90(23):10513-10526. doi: 10.1128/JVI.01532-16. Print 2016 Dec 1. J Virol. 2016. PMID: 27630246 Free PMC article.
References
-
- Abraham N, Miceli M C, Parnes J R, Veillette A. Enhancement of T-cell responsiveness by the lymphocyte-specific tyrosine kinase p56lck. Nature (London) 1991;350:62–66. - PubMed
-
- Anderson P, Blue M-L, Morimoto C, Schlossman S F. Cross-linking of T3 (CD3) with T4 (CD4) enhances the proliferation of resting T lymphocytes. J Immunol. 1987;139:678–682. - PubMed
-
- Baldari C T, Pelicci G, Di Somma M M, Milia E, Giuli S, Pelicci P G, Telford J L. CD4 triggering results in tyrosine phosphorylation of the Shc adaptor protein. Oncogene. 1995;10:1141–1147. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

