Effects of substituting granulin or a granulin-polyhedrin chimera for polyhedrin on virion occlusion and polyhedral morphology in Autographa californica multinucleocapsid nuclear polyhedrosis virus
- PMID: 9621097
- PMCID: PMC110449
- DOI: 10.1128/JVI.72.7.6237-6243.1998
Effects of substituting granulin or a granulin-polyhedrin chimera for polyhedrin on virion occlusion and polyhedral morphology in Autographa californica multinucleocapsid nuclear polyhedrosis virus
Abstract
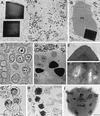
Substitution of granulin from the Trichoplusia ni granulosis virus (TnGV) for polyhedrin of the Autographa californica multinucleocapsid nuclear polyhedrosis virus (AcMNPV) yielded a few very large (2 to 5 micron) cuboidal inclusions in the cytoplasm and nucleus of infected cells. These polyhedra lacked the beveled edges characteristic of wild-type AcMNPV polyhedra, contained fractures, and occluded few virions. Placing a nuclear localization signal (KRKK) in granulin directed more granulin to the nucleus and resulted in more structurally uniform cuboidal inclusions in which no virions were observed. A granulin-polyhedrin chimera produced tetrahedral occlusions with more virions than granulin inclusions but many fewer than wild-type polyhedra. Despite the unusual structure of the granulin and granulin-polyhedrin inclusions, they interacted with AcMNPV p10 fibrillar structures and electron-dense spacers that are precursors of the polyhedral calyx. The change in inclusion shape obtained with the granulin-polyhedrin chimera demonstrates that the primary amino acid sequence affects occlusion body shape, but the large cuboidal inclusions formed by granulin indicate that the amino acid sequence is not the only determinant. The failure of granulin or the granulin-polyhedrin chimera to properly occlude AcMNPV virions suggests that specific interactions occur between polyhedrin and other viral proteins which facilitate normal virion occlusion and occlusion body assembly and shape in baculoviruses.
Figures


Similar articles
-
Specificity of polyhedrin in the generation of baculovirus occlusion bodies.J Gen Virol. 1999 Apr;80 ( Pt 4):1045-1053. doi: 10.1099/0022-1317-80-4-1045. J Gen Virol. 1999. PMID: 10211975
-
Characterization of a virion occlusion-defective Autographa californica multiple nucleopolyhedrovirus mutant lacking the p26, p10 and p74 genes.J Gen Virol. 2009 Jul;90(Pt 7):1641-1648. doi: 10.1099/vir.0.010397-0. Epub 2009 Mar 4. J Gen Virol. 2009. PMID: 19264658
-
Cloning and sequencing of the granulin gene from the Trichoplusia ni granulosis virus.Virology. 1985 Mar;141(2):328-32. doi: 10.1016/0042-6822(85)90267-3. Virology. 1985. PMID: 4082501
-
Polyhedrin sequence determines the tetrahedral shape of occlusion bodies in Thysanoplusia orichalcea single-nucleocapsid nucleopolyhedrovirus.J Gen Virol. 1998 Oct;79 ( Pt 10):2549-56. doi: 10.1099/0022-1317-79-10-2549. J Gen Virol. 1998. PMID: 9780063
-
Enhancement of polyhedrin nuclear localization during baculovirus infection.J Virol. 1992 Dec;66(12):6903-11. doi: 10.1128/JVI.66.12.6903-6911.1992. J Virol. 1992. PMID: 1433499 Free PMC article.
Cited by
-
Identification of novel residues involved in nuclear localization of a baculovirus polyhedrin protein.Virus Genes. 2000 Oct;21(3):233-40. doi: 10.1023/a:1008151916849. Virus Genes. 2000. PMID: 11129641
-
Identification of domains in Autographa californica multiple nucleopolyhedrovirus late expression factor 3 required for nuclear transport of P143.J Virol. 2005 Sep;79(17):10915-22. doi: 10.1128/JVI.79.17.10915-10922.2005. J Virol. 2005. PMID: 16103143 Free PMC article.
-
Baculovirus transregulator IE1 requires a dimeric nuclear localization element for nuclear import and promoter activation.J Virol. 2002 Sep;76(18):9505-15. doi: 10.1128/jvi.76.18.9505-9515.2002. J Virol. 2002. PMID: 12186932 Free PMC article.
-
A Novel Betabaculovirus Isolated from the Monocot Pest Mocis latipes (Lepidoptera: Noctuidae) and the Evolution of Multiple-Copy Genes.Viruses. 2018 Mar 16;10(3):134. doi: 10.3390/v10030134. Viruses. 2018. PMID: 29547534 Free PMC article.
-
Molecular characterization of Bombyx mori cytoplasmic polyhedrosis virus genome segment 4.J Virol. 2001 Jan;75(2):988-95. doi: 10.1128/JVI.75.2.988-995.2001. J Virol. 2001. PMID: 11134312 Free PMC article.
References
-
- Akiyoshi D, Chakerian R, Rohrmann G F, Nesson M H, Beaudreau G S. Cloning and sequencing of the granulin gene from the Trichoplusia ni granulosis virus. Virology. 1985;141:328–332. - PubMed
-
- Arnott H J, Smith K M. An ultrastructural study of the development of a granulosis virus in the cells of the moth Plodia interpunctella (Hbn.) J Ultrastruct Res. 1968;21:251–268. - PubMed
-
- Brown M, Faulkner P, Cochran M A, Chung K L. Characterization of two morphology mutants of Autographa californica nuclear polyhedrosis virus with large cuboidal inclusion bodies. J Gen Virol. 1980;50:309–316.
-
- Carstens E B, Lin-Bai Y, Faulkner P. A point mutation in the polyhedrin gene of a baculovirus, Autographa californica MNPV, prevents crystallization of occlusion bodies. J Gen Virol. 1987;68:901–905. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

