An Arabidopsis VPS45p homolog implicated in protein transport to the vacuole
- PMID: 9625693
- PMCID: PMC34960
- DOI: 10.1104/pp.117.2.407
An Arabidopsis VPS45p homolog implicated in protein transport to the vacuole
Abstract
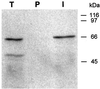
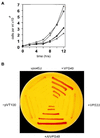
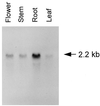
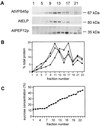
The Sec1p family of proteins is required for vesicle-mediated protein trafficking between various organelles of the endomembrane system. This family includes Vps45p, which is required for transport to the vacuole in yeast (Saccharomyces cerevisiae). We have isolated a cDNA encoding a VPS45 homolog from Arabidopsis thaliana (AtVPS45). The cDNA is able to complement both the temperature-sensitive growth defect and the vacuolar-targeting defect of a yeast vps45 mutant, indicating that the two proteins are functionally related. AtVPS45p is a peripheral membrane protein that associates with microsomal membranes. Sucrose-density gradient fractionation demonstrated that AtVPS45p co-fractionates with AtELP, a potential vacuolar protein sorting receptor, implying that they may reside on the same membrane populations. These results indicate that AtVPS45p is likely to function in the transport of proteins to the vacuole in plants.
Figures


References
-
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

