Auxin deprivation induces synchronous Golgi differentiation in suspension-cultured tobacco BY-2 cells
- PMID: 9625703
- PMCID: PMC34970
- DOI: 10.1104/pp.117.2.501
Auxin deprivation induces synchronous Golgi differentiation in suspension-cultured tobacco BY-2 cells
Abstract
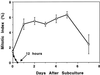
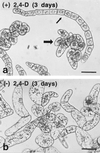
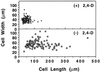
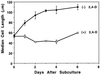
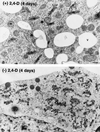
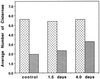
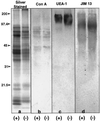
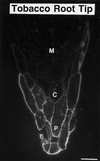
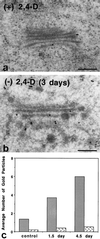
To date, the lack of a method for inducing plant cells and their Golgi stacks to differentiate in a synchronous manner has made it difficult to characterize the nature and extent of Golgi retailoring in biochemical terms. Here we report that auxin deprivation can be used to induce a uniform population of suspension-cultured tobacco (Nicotiana tabacum cv BY-2) cells to differentiate synchronously during a 4-d period. Upon removal of auxin, the cells stop dividing, undergo elongation, and differentiate in a manner that mimics the formation of slime-secreting epidermal and peripheral root-cap cells. The morphological changes to the Golgi apparatus include a proportional increase in the number of trans-Golgi cisternae, a switch to larger-sized secretory vesicles that bud from the trans-Golgi cisternae, and an increase in osmium staining of the secretory products. Biochemical alterations include an increase in large, fucosylated, mucin-type glycoproteins, changes in the types of secreted arabinogalactan proteins, and an increase in the amounts and types of molecules containing the peripheral root-cap-cell-specific epitope JIM 13. Taken together, these findings support the hypothesis that auxin deprivation can be used to induce tobacco BY-2 cells to differentiate synchronously into mucilage-secreting cells.
Figures




References
-
- Blum EA, Beier H, Gross HJ. Improved silver staining of plant proteins, RNA, and DNA in polyacrylamide gels. Electrophoresis. 1987;8:93–99.
-
- Craig S, Staehelin LA. High pressure freezing of intact plant tissues: evaluation and characterization of novel features of the endoplasmic reticulum and associated membrane systems. Eur J Cell Biol. 1988;46:80–93. - PubMed
-
- Cunninghame ME, Hall JL. A quantitative stereological analysis of the effect of indoleacetic acid on the dictyosomes in pea stem epidermal cells. Protoplasma. 1985;125:230–234.
-
- Driouich A, Levy S, Staehelin LA, Faye L. Structural and functional organization of the Golgi apparatus in plant cells. Plant Physiol Biochem. 1994;32:731–749.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

