The NAD(P)H dehydrogenase in barley thylakoids is photoactivatable and uses NADPH as well as NADH
- PMID: 9625705
- PMCID: PMC34972
- DOI: 10.1104/pp.117.2.525
The NAD(P)H dehydrogenase in barley thylakoids is photoactivatable and uses NADPH as well as NADH
Abstract
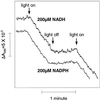
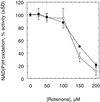
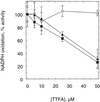
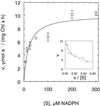
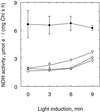
An improved light-dependent assay was used to characterize the NAD(P)H dehydrogenase (NDH) in thylakoids of barley (Hordeum vulgare L.). The enzyme was sensitive to rotenone, confirming the involvement of a complex I-type enzyme. NADPH and NADH were equally good substrates for the dehydrogenase. Maximum rates of activity were 10 to 19 &mgr;mol electrons mg-1 chlorophyll h-1, corresponding to about 3% of linear electron-transport rates, or to about 40% of ferredoxin-dependent cyclic electron-transport rates. The NDH was activated by light treatment. After photoactivation, a subsequent light-independent period of about 1 h was required for maximum activation. The NDH could also be activated by incubation of the thylakoids in low-ionic-strength buffer. The kinetics, substrate specificity, and inhibitor profiles were essentially the same for both induction strategies. The possible involvement of ferredoxin:NADP+ oxidoreductase (FNR) in the NDH activity could be excluded based on the lack of preference for NADPH over NADH. Furthermore, thenoyltrifluoroacetone inhibited the diaphorase activity of FNR but not the NDH activity. These results also lead to the conclusion that direct reduction of plastoquinone by FNR is negligible.
Figures


Similar articles
-
Involvement of the thylakoidal NADH-plastoquinone-oxidoreductase complex in the early responses to ozone exposure of barley (Hordeum vulgare L.) seedlings.J Exp Bot. 2005 Jan;56(409):205-18. doi: 10.1093/jxb/eri024. Epub 2004 Nov 22. J Exp Bot. 2005. PMID: 15557294
-
Reduction of the plastoquinone pool by exogenous NADH and NADPH in higher plant chloroplasts. Characterization of a NAD(P)H-plastoquinone oxidoreductase activity.Biochim Biophys Acta. 1998 Jan 27;1363(1):59-69. doi: 10.1016/s0005-2728(97)00074-1. Biochim Biophys Acta. 1998. PMID: 9526046
-
Enzymatic characterization of an active NDH complex from Thermosynechococcus elongatus.FEBS Lett. 2013 Aug 2;587(15):2340-5. doi: 10.1016/j.febslet.2013.05.040. Epub 2013 May 27. FEBS Lett. 2013. PMID: 23722112
-
Chloroplast NDH: A different enzyme with a structure similar to that of respiratory NADH dehydrogenase.Biochim Biophys Acta. 2016 Jul;1857(7):1015-22. doi: 10.1016/j.bbabio.2015.10.013. Epub 2015 Oct 28. Biochim Biophys Acta. 2016. PMID: 26519774 Review.
-
The plastid NAD(P)H dehydrogenase-like complex: structure, function and evolutionary dynamics.Biochem J. 2019 Oct 15;476(19):2743-2756. doi: 10.1042/BCJ20190365. Biochem J. 2019. PMID: 31654059 Review.
Cited by
-
Alternative photosystem I-driven electron transport routes: mechanisms and functions.Photosynth Res. 2004;82(1):17-33. doi: 10.1023/B:PRES.0000040442.59311.72. Photosynth Res. 2004. PMID: 16228610
-
Photoinhibition of Photosystem I in field-grown barley (Hordeum vulgare L.): Induction, recovery and acclimation.Photosynth Res. 2000;64(1):53-61. doi: 10.1023/A:1026524302191. Photosynth Res. 2000. PMID: 16228443
-
Photo-induction of an NADPH dehydrogenase which functions as a mediator of electron transport to the intersystem chain in the cyanobacterium Synechocystis PCC6803.Photosynth Res. 2001;70(2):167-73. doi: 10.1023/A:1017946524199. Photosynth Res. 2001. PMID: 16228350
-
Alternative electron transports participate in the maintenance of violaxanthin De-epoxidase activity of Ulva sp. under low irradiance.PLoS One. 2013 Nov 8;8(11):e78211. doi: 10.1371/journal.pone.0078211. eCollection 2013. PLoS One. 2013. PMID: 24250793 Free PMC article.
-
Mercury inhibits the non-photochemical reduction of plastoquinone by exogenous NADPH and NADH: evidence from measurements of the polyphasic chlorophyll a fluorescence rise in spinach chloroplasts.Photosynth Res. 2002;74(1):37-50. doi: 10.1023/A:1020884500821. Photosynth Res. 2002. PMID: 16228543
References
-
- Andersen B, Koch B, Scheller HV. Structural and functional analysis of the reducing side of photosystem I. Physiol Plant. 1992;84:154–161.
-
- Appel J, Schulz R. Sequence analysis of an operon of a NAD(P)-reducing nickel hydrogenase from the cyanobacterium Synechocystis sp. PCC 6803 gives additional evidence for direct coupling of the enzyme to NAD(P)H-dehydrogenase (complex I) Biochim Biophys Acta. 1996;1298:141–147. - PubMed
-
- Berger S, Ellersiek U, Kinzelt D, Steinmüller K. Immunopurification of a subcomplex of the NAD(P)H-plastoquinone-oxidoreductase from the cyanobacterium Synechocystis sp. PCC6803. FEBS Lett. 1993;326:246–250. - PubMed
LinkOut - more resources
Full Text Sources

