The NAD(P)H dehydrogenase in barley thylakoids is photoactivatable and uses NADPH as well as NADH
- PMID: 9625705
- PMCID: PMC34972
- DOI: 10.1104/pp.117.2.525
The NAD(P)H dehydrogenase in barley thylakoids is photoactivatable and uses NADPH as well as NADH
Abstract
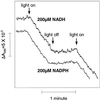
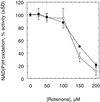
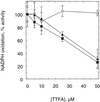
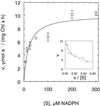
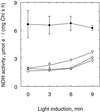
An improved light-dependent assay was used to characterize the NAD(P)H dehydrogenase (NDH) in thylakoids of barley (Hordeum vulgare L.). The enzyme was sensitive to rotenone, confirming the involvement of a complex I-type enzyme. NADPH and NADH were equally good substrates for the dehydrogenase. Maximum rates of activity were 10 to 19 &mgr;mol electrons mg-1 chlorophyll h-1, corresponding to about 3% of linear electron-transport rates, or to about 40% of ferredoxin-dependent cyclic electron-transport rates. The NDH was activated by light treatment. After photoactivation, a subsequent light-independent period of about 1 h was required for maximum activation. The NDH could also be activated by incubation of the thylakoids in low-ionic-strength buffer. The kinetics, substrate specificity, and inhibitor profiles were essentially the same for both induction strategies. The possible involvement of ferredoxin:NADP+ oxidoreductase (FNR) in the NDH activity could be excluded based on the lack of preference for NADPH over NADH. Furthermore, thenoyltrifluoroacetone inhibited the diaphorase activity of FNR but not the NDH activity. These results also lead to the conclusion that direct reduction of plastoquinone by FNR is negligible.
Figures


References
-
- Andersen B, Koch B, Scheller HV. Structural and functional analysis of the reducing side of photosystem I. Physiol Plant. 1992;84:154–161.
-
- Appel J, Schulz R. Sequence analysis of an operon of a NAD(P)-reducing nickel hydrogenase from the cyanobacterium Synechocystis sp. PCC 6803 gives additional evidence for direct coupling of the enzyme to NAD(P)H-dehydrogenase (complex I) Biochim Biophys Acta. 1996;1298:141–147. - PubMed
-
- Berger S, Ellersiek U, Kinzelt D, Steinmüller K. Immunopurification of a subcomplex of the NAD(P)H-plastoquinone-oxidoreductase from the cyanobacterium Synechocystis sp. PCC6803. FEBS Lett. 1993;326:246–250. - PubMed
LinkOut - more resources
Full Text Sources

