Desensitization of the perception system for chitin fragments in tomato cells
- PMID: 9625717
- PMCID: PMC34984
- DOI: 10.1104/pp.117.2.643
Desensitization of the perception system for chitin fragments in tomato cells
Abstract
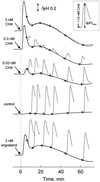
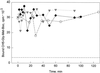
Suspension-cultured tomato (Lycopersicon esculentum) cells react to stimulation by chitin fragments with a rapid, transient alkalinization of the growth medium, but behave refractory to a second treatment with the same stimulus (G. Felix, M. Regenass, T. Boller [1993] Plant J 4: 307-316). We analyzed this phenomenon and found that chitin fragments caused desensitization in a time- and concentration-dependent manner. Partially desensitized cells exhibited a clear shift toward lower sensitivity of the perception system. The ability of chitin oligomers to induce desensitization depended on the degree of polymerization (DP), with DP5 approximately DP4 >> DP3 >> DP2 > DP1. This correlates with the ability of these oligomers to induce the alkalinization response and to compete for the high-affinity binding site on tomato cells and microsomal membranes, indicating that the alkalinization response and the desensitization process are mediated by the same receptor. The dose required for half-maximal desensitization was about 20 times lower than the dose required for half-maximal alkalinization; desensitization could therefore be used as a highly sensitive bioassay for chitin fragments and chitin-related stimuli such as lipochitooligosaccharides (nodulation factors) from Rhizobium leguminosarum. Desensitization was not associated with increased inactivation of the stimulus or with a disappearance of high-affinity binding sites from the cell surface, and thus appears to be caused by an intermediate step in signal transduction.
Figures





Similar articles
-
Specific, high affinity binding of chitin fragments to tomato cells and membranes. Competitive inhibition of binding by derivatives of chitooligosaccharides and a Nod factor of Rhizobium.J Biol Chem. 1994 Jul 8;269(27):17931-8. J Biol Chem. 1994. PMID: 8027050
-
Perception of Fungal Sterols in Plants (Subnanomolar Concentrations of Ergosterol Elicit Extracellular Alkalinization in Tomato Cells).Plant Physiol. 1995 Feb;107(2):485-490. doi: 10.1104/pp.107.2.485. Plant Physiol. 1995. PMID: 12228375 Free PMC article.
-
Alfalfa and tobacco cells react differently to chitin oligosaccharides and sinorhizobium meliloti nodulation factors.Planta. 1999 Nov;210(1):157-64. doi: 10.1007/s004250050665. Planta. 1999. PMID: 10592044
-
Perception of Rhizobium nodulation factors by tomato cells and inactivation by root chitinases.Proc Natl Acad Sci U S A. 1994 Mar 15;91(6):2196-200. doi: 10.1073/pnas.91.6.2196. Proc Natl Acad Sci U S A. 1994. PMID: 8134372 Free PMC article.
-
N-Acetyl-D-glucosamine oligosaccharides induce mucin secretion from colonic tissue and induce differentiation of human keratinocytes.J Pharm Pharmacol. 2008 Feb;60(2):197-204. doi: 10.1211/jpp.60.2.0008. J Pharm Pharmacol. 2008. PMID: 18237467
Cited by
-
The right microbe-associated molecular patterns for effective recognition by plants.Front Microbiol. 2022 Sep 26;13:1019069. doi: 10.3389/fmicb.2022.1019069. eCollection 2022. Front Microbiol. 2022. PMID: 36225366 Free PMC article. Review.
-
Convergence of signaling pathways induced by systemin, oligosaccharide elicitors, and ultraviolet-B radiation at the level of mitogen-activated protein kinases in Lycopersicon peruvianum suspension-cultured cells.Plant Physiol. 2003 Aug;132(4):1728-38. doi: 10.1104/pp.103.024414. Plant Physiol. 2003. PMID: 12913131 Free PMC article.
-
Chitin and chitin-related compounds in plant-fungal interactions.Mycology. 2018 May 15;9(3):189-201. doi: 10.1080/21501203.2018.1473299. eCollection 2018. Mycology. 2018. PMID: 30181925 Free PMC article. Review.
-
Linear beta-1,3 glucans are elicitors of defense responses in tobacco.Plant Physiol. 2000 Nov;124(3):1027-38. doi: 10.1104/pp.124.3.1027. Plant Physiol. 2000. PMID: 11080280 Free PMC article.
-
The grapevine LysM receptor-like kinase VvLYK5-1 recognizes chitin oligomers through its association with VvLYK1-1.Front Plant Sci. 2023 Feb 2;14:1130782. doi: 10.3389/fpls.2023.1130782. eCollection 2023. Front Plant Sci. 2023. PMID: 36818830 Free PMC article.
References
-
- Armitage JP. Behavioral responses in bacteria. Annu Rev Physiol. 1992;54:683–714. - PubMed
-
- Basse CW, Bock K, Boller T. Elicitors and suppressors of the defense response in tomato cells: purification and characterization of glycopeptide elicitors and glycan suppressors generated by enzymatic cleavage of yeast invertase. J Biol Chem. 1992;267:10258–10265. - PubMed
-
- Basse CW, Fath A, Boller T. High affinity binding of glycopeptide elicitor to tomato cells and microsomal membranes and displacement by specific glycan suppressors. J Biol Chem. 1993;268:14724–14731. - PubMed
-
- Baureithel K, Felix G, Boller T. Specific, high affinity binding of chitin fragments to tomato cells and membranes: competitive inhibition of binding by derivatives of chitooligosaccharides and a Nod factor of Rhizobium. J Biol Chem. 1994;269:17931–17938. - PubMed
-
- Boller T. Chemoperception of microbial signals in plant cells. Annu Rev Plant Physiol Plant Mol Biol. 1995;46:189–214.
LinkOut - more resources
Full Text Sources

