Desensitization of the perception system for chitin fragments in tomato cells
- PMID: 9625717
- PMCID: PMC34984
- DOI: 10.1104/pp.117.2.643
Desensitization of the perception system for chitin fragments in tomato cells
Abstract
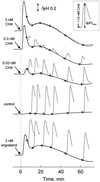
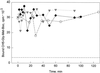
Suspension-cultured tomato (Lycopersicon esculentum) cells react to stimulation by chitin fragments with a rapid, transient alkalinization of the growth medium, but behave refractory to a second treatment with the same stimulus (G. Felix, M. Regenass, T. Boller [1993] Plant J 4: 307-316). We analyzed this phenomenon and found that chitin fragments caused desensitization in a time- and concentration-dependent manner. Partially desensitized cells exhibited a clear shift toward lower sensitivity of the perception system. The ability of chitin oligomers to induce desensitization depended on the degree of polymerization (DP), with DP5 approximately DP4 >> DP3 >> DP2 > DP1. This correlates with the ability of these oligomers to induce the alkalinization response and to compete for the high-affinity binding site on tomato cells and microsomal membranes, indicating that the alkalinization response and the desensitization process are mediated by the same receptor. The dose required for half-maximal desensitization was about 20 times lower than the dose required for half-maximal alkalinization; desensitization could therefore be used as a highly sensitive bioassay for chitin fragments and chitin-related stimuli such as lipochitooligosaccharides (nodulation factors) from Rhizobium leguminosarum. Desensitization was not associated with increased inactivation of the stimulus or with a disappearance of high-affinity binding sites from the cell surface, and thus appears to be caused by an intermediate step in signal transduction.
Figures





References
-
- Armitage JP. Behavioral responses in bacteria. Annu Rev Physiol. 1992;54:683–714. - PubMed
-
- Basse CW, Bock K, Boller T. Elicitors and suppressors of the defense response in tomato cells: purification and characterization of glycopeptide elicitors and glycan suppressors generated by enzymatic cleavage of yeast invertase. J Biol Chem. 1992;267:10258–10265. - PubMed
-
- Basse CW, Fath A, Boller T. High affinity binding of glycopeptide elicitor to tomato cells and microsomal membranes and displacement by specific glycan suppressors. J Biol Chem. 1993;268:14724–14731. - PubMed
-
- Baureithel K, Felix G, Boller T. Specific, high affinity binding of chitin fragments to tomato cells and membranes: competitive inhibition of binding by derivatives of chitooligosaccharides and a Nod factor of Rhizobium. J Biol Chem. 1994;269:17931–17938. - PubMed
-
- Boller T. Chemoperception of microbial signals in plant cells. Annu Rev Plant Physiol Plant Mol Biol. 1995;46:189–214.
LinkOut - more resources
Full Text Sources

