The kinetics of zeaxanthin formation is retarded by dicyclohexylcarbodiimide
- PMID: 9625719
- PMCID: PMC34986
- DOI: 10.1104/pp.117.2.659
The kinetics of zeaxanthin formation is retarded by dicyclohexylcarbodiimide
Abstract
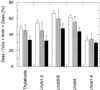
The de-epoxidation of violaxanthin to antheraxanthin (Anth) and zeaxanthin (Zeax) in the xanthophyll cycle of higher plants and the generation of nonphotochemical fluorescence quenching in the antenna of photosystem II (PSII) are induced by acidification of the thylakoid lumen. Dicyclohexylcarbodiimide (DCCD) has been shown (a) to bind to lumen-exposed carboxy groups of antenna proteins and (b) to inhibit the pH-dependent fluorescence quenching. The possible influence of DCCD on the de-epoxidation reactions has been investigated in isolated pea (Pisum sativum L.) thylakoids. The Zeax formation was found to be slowed down in the presence of DCCD. The second step (Anth --> Zeax) of the reaction sequence seemed to be more affected than the violaxanthin --> Anth conversion. Comparative studies with antenna-depleted thylakoids from plants grown under intermittent light and with unstacked thylakoids were in agreement with the assumption that binding of DCCD to antenna proteins is probably responsible for the retarded kinetics. Analyses of the DCCD-induced alterations in different antenna subcomplexes showed that Zeax formation in the PSII antenna proteins was predominantly influenced by DCCD, whereas Zeax formation in photosystem I was nearly unaffected. Our data support the suggestion that DCCD binding to PSII antenna proteins is responsible for the observed alterations in xanthophyll conversion.
Figures

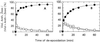

Similar articles
-
Dynamics of Xanthophyll-Cycle Activity in Different Antenna Subcomplexes in the Photosynthetic Membranes of Higher Plants (The Relationship between Zeaxanthin Conversion and Nonphotochemical Fluorescence Quenching).Plant Physiol. 1997 Dec;115(4):1609-1618. doi: 10.1104/pp.115.4.1609. Plant Physiol. 1997. PMID: 12223884 Free PMC article.
-
The xanthophyll cycle of higher plants: influence of antenna size and membrane organization.Biochim Biophys Acta. 1998 Jan 27;1363(1):47-58. doi: 10.1016/s0005-2728(97)00093-5. Biochim Biophys Acta. 1998. PMID: 9526041
-
A theoretical investigation of xanthophyll-protein hydrogen bonding in the photosystem II antenna.J Phys Chem B. 2012 Apr 12;116(14):4310-8. doi: 10.1021/jp206179c. Epub 2012 Apr 3. J Phys Chem B. 2012. PMID: 22439795
-
Xanthophyll cycle-dependent nonphotochemical quenching in Photosystem II: Mechanistic insights gained from Arabidopsis thaliana L. mutants that lack violaxanthin deepoxidase activity and/or lutein.Photosynth Res. 2001;67(1-2):89-101. doi: 10.1023/A:1010657000548. Photosynth Res. 2001. PMID: 16228319
-
Mechanism and regulation of the violaxanthin cycle: the role of antenna proteins and membrane lipids.Biochim Biophys Acta. 2009 Jan;1787(1):3-14. doi: 10.1016/j.bbabio.2008.09.013. Epub 2008 Oct 11. Biochim Biophys Acta. 2009. PMID: 18976630 Review.
Cited by
-
Greening under high light or cold temperature affects the level of xanthophyll-cycle pigments, early light-inducible proteins, and light-harvesting polypeptides in wild-type barley and the chlorina f2 mutant.Plant Physiol. 1999 May;120(1):193-204. doi: 10.1104/pp.120.1.193. Plant Physiol. 1999. PMID: 10318697 Free PMC article.
-
Characterization of acclimation of Hordeum vulgare to high irradiation based on different responses of photosynthetic activity and pigment composition.Photosynth Res. 2002;72(1):71-83. doi: 10.1023/A:1016018900535. Photosynth Res. 2002. PMID: 16228536
-
In vivo photoprotection mechanisms observed from leaf spectral absorbance changes showing VIS-NIR slow-induced conformational pigment bed changes.Photosynth Res. 2019 Dec;142(3):283-305. doi: 10.1007/s11120-019-00664-3. Epub 2019 Sep 20. Photosynth Res. 2019. PMID: 31541418 Free PMC article.
References
-
- Aro E-M, Virgin I, Andersson B. Photoinhibition of photosystem II: inactivation, protein damage and turnover. Biochim Biophys Acta. 1993;1143:113–134. - PubMed
-
- Arvidsson P-O, Bratt CE, Carlsson M, Åkerlund H-E. Purification and identification of the violaxanthin deepoxidase as a 43 kDa protein. Photosynth Res. 1996;49:119–129. - PubMed
-
- Azzi A, Casey RP, Nalecz MJ. The effect of N,N′-dicyclohexylcarbodiimide on enzymes of bioenergetic relevance. Biochim Biophys Acta. 1984;768:209–226. - PubMed
-
- Bassi R, Pineau B, Dainese P, Marquardt J. Carotenoid-binding proteins of photosystem II. Eur J Biochem. 1993;212:297–303. - PubMed
-
- Bratt CE, Arvidsson P-O, Carlsson M, Åkerlund H-E. Regulation of violaxanthin de-epoxidase activity by pH and ascorbate concentration. Photosynth Res. 1995;45:169–175. - PubMed
LinkOut - more resources
Full Text Sources

