Effect of water stress on cell division and cell-division-cycle 2-like cell-cycle kinase activity in wheat leaves
- PMID: 9625720
- PMCID: PMC34987
- DOI: 10.1104/pp.117.2.667
Effect of water stress on cell division and cell-division-cycle 2-like cell-cycle kinase activity in wheat leaves
Abstract
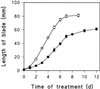
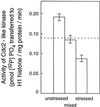
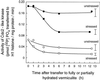
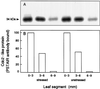
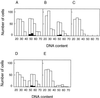
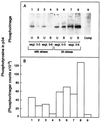
In wheat (Triticum aestivum) seedlings subjected to a mild water stress (water potential of -0.3 MPa), the leaf-elongation rate was reduced by one-half and the mitotic activity of mesophyll cells was reduced to 42% of well-watered controls within 1 d. There was also a reduction in the length of the zone of mesophyll cell division to within 4 mm from the base compared with 8 mm in control leaves. However, the period of division continued longer in the stressed than in the control leaves, and the final cell number in the stressed leaves reached 85% of controls. Cyclin-dependent protein kinase enzymes that are required in vivo for DNA replication and mitosis were recovered from the meristematic zone of leaves by affinity for p13(suc1). Water stress caused a reduction in H1 histone kinase activity to one-half of the control level, although amounts of the enzyme were unaffected. Reduced activity was correlated with an increased proportion of the 34-kD Cdc2-like kinase (an enzyme sharing with the Cdc2 protein of other eukaryotes the same size, antigenic sites, affinity for p13(suc1), and H1 histone kinase catalytic activity) deactivated by tyrosine phosphorylation. Deactivation to 50% occurred within 3 h of stress imposition in cells at the base of the meristematic zone and was therefore too fast to be explained by a reduction in the rate at which cells reached mitosis because of slowing of growth; rather, stress must have acted more immediately on the enzyme. The operation of controls slowing the exit from the G1 and G2 phases is discussed. We suggest that a water-stress signal acts on Cdc2 kinase by increasing phosphorylation of tyrosine, causing a shift to the inhibited form and slowing cell production.
Figures



References
-
- Beemster GTS, Masle J, Williamson RE, Farquhar GD. Effects of soil resistance to root penetration on leaf expansion in wheat (Triticum aestivum L.): kinematic analysis of leaf elongation. J Exp Bot. 1997;47:1663–1678.
-
- Bergounioux C, Perennes C, Hemerly AS, Qin LX, Sarda C, Inzé D, Gadal P. Relation between protoplast division, cell-cycle stage and nuclear chromatin structure. Protoplasma. 1988;142:127–136.
-
- Bitonti MB, Ferraro F, Floris C, Innocenti AM. Response of meristematic cells to osmotic stress in Triticum durum. Biochem Physiol Pflanz. 1991;187:453–457.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

