Wound signaling in tomato plants. Evidence that aba is not a primary signal for defense gene activation
- PMID: 9625722
- PMCID: PMC34989
- DOI: 10.1104/pp.117.2.687
Wound signaling in tomato plants. Evidence that aba is not a primary signal for defense gene activation
Abstract
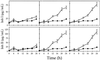
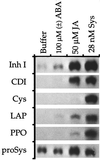
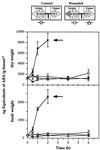
The effects of abscisic acid (ABA) on the accumulation of proteinase inhibitors I (Inh I) and II (Inh II) in young, excised tomato (Lycopersicon esculentum L.) plants were investigated. When supplied to excised plants through the cut stems, 100 &mgr;m ABA induced the activation of the ABA-responsive le4 gene. However, under the same conditions of assay, ABA at concentrations of up to 100 &mgr;m induced only low levels of proteinase-inhibitor proteins or mRNAs, compared with levels induced by systemin or jasmonic acid over the 24 h following treatment. In addition, ABA only weakly induced the accumulation of mRNAs of several other wound-response proteins. Assays of the ABA concentrations in leaves following wounding indicated that the ABA levels increased preferentially near the wound site, suggesting that ABA may have accumulated because of desiccation. The evidence suggests that ABA is not a component of the wound-inducible signal transduction pathway leading to defense gene activation but is likely involved in the general maintenance of a healthy plant physiology that facilitates a normal wound response.
Figures




References
-
- Bishop PD, Pearce G, Bryant JE, Ryan CA. Isolation and characterization of the proteinase inhibitor-inducing factor from tomato leaves: identity and activity of poly- and oligogalacturonide fragments. J Biol Chem. 1984;259:13172–13176. - PubMed
-
- Cohen A, Bray EA. Characterization of three mRNAs that accumulate in wilted tomato leaves in response to elevated levels of endogenous abscisic acid. Planta. 1990;182:27–33. - PubMed
LinkOut - more resources
Full Text Sources

