Extrathymic T cell deletion and allogeneic stem cell engraftment induced with costimulatory blockade is followed by central T cell tolerance
- PMID: 9625763
- PMCID: PMC2212372
- DOI: 10.1084/jem.187.12.2037
Extrathymic T cell deletion and allogeneic stem cell engraftment induced with costimulatory blockade is followed by central T cell tolerance
Abstract
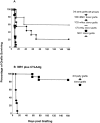
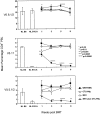
A reliable, nontoxic method of inducing transplantation tolerance is needed to overcome the problems of chronic organ graft rejection and immunosuppression-related toxicity. Treatment of mice with single injections of an anti-CD40 ligand antibody and CTLA4Ig, a low dose (3 Gy) of whole body irradiation, plus fully major histocompatibility complex-mismatched allogeneic bone marrow transplantation (BMT) reliably induced high levels (>40%) of stable (>8 mo) multilineage donor hematopoiesis. Chimeric mice permanently accepted donor skin grafts (>100 d), and rapidly rejected third party grafts. Progressive deletion of donor-reactive host T cells occurred among peripheral CD4(+) lymphocytes, beginning as early as 1 wk after bone marrow transplantation. Early deletion of peripheral donor-reactive host CD4 cells also occurred in thymectomized, similarly treated marrow recipients, demonstrating a role for peripheral clonal deletion of donor-reactive T cells after allogeneic BMT in the presence of costimulatory blockade. Central intrathymic deletion of newly developing T cells ensued after donor stem cell engraftment had occurred. Thus, we have shown that high levels of chimerism and systemic T cell tolerance can be reliably achieved without myeloablation or T cell depletion of the host. Chronic immunosuppression and rejection are avoided with this powerful, nontoxic approach to inducing tolerance.
Figures



References
-
- Gjertson, D.W. 1991. Survival trends in long-term first cadaver-donor kidney transplants. In Clinical Transplants. P.I. Terasaki and J.M. Cecka, editors. UCLA Tissue Typing Laboratory, Los Angeles. 225–235. - PubMed
-
- Linsley PS, Ledbetter JA. The role of the CD28 receptor during T cell responses to antigen. Annu Rev Immunol. 1993;11:191–212. - PubMed
-
- Foy TM, Aruffo A, Bajorath J, Buhlmann JE, Noelle RJ. Immune regulation by CD40 and its ligand gp39. Annu Rev Immunol. 1996;14:591–617. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

