NKp44, a novel triggering surface molecule specifically expressed by activated natural killer cells, is involved in non-major histocompatibility complex-restricted tumor cell lysis
- PMID: 9625766
- PMCID: PMC2212362
- DOI: 10.1084/jem.187.12.2065
NKp44, a novel triggering surface molecule specifically expressed by activated natural killer cells, is involved in non-major histocompatibility complex-restricted tumor cell lysis
Abstract
After culture in interleukin (IL)-2, natural killer (NK) cells acquire an increased capability of mediating non-major histocompatibility complex (MHC)-restricted tumor cell lysis. This may reflect, at least in part, the de novo expression by NK cells of triggering receptors involved in cytolysis. In this study we identified a novel 44-kD surface molecule (NKp44) that is absent in freshly isolated peripheral blood lymphocytes but is progressively expressed by all NK cells in vitro after culture in IL-2. Different from other markers of cell activation such as CD69 or VLA.2, NKp44 is absent in activated T lymphocytes or T cell clones. Since NKp44 was not detected in any of the other cell lineages analyzed, it appears as the first marker specific for activated human NK cells. Monoclonal antibody (mAb)-mediated cross-linking of NKp44 in cloned NK cells resulted in strong activation of target cell lysis in a redirected killing assay. This data indicated that NKp44 can mediate triggering of NK cell cytotoxicity. mAb-mediated masking of NKp44 resulted in partial inhibition of cytolytic activity against certain (FcgammaR-negative) NK-susceptible target cells. This inhibition was greatly increased by the simultaneous masking of p46, another recently identified NK-specific triggering surface molecule. These data strongly suggest that NKp44 functions as a triggering receptor selectively expressed by activated NK cells that, together with p46, may be involved in the process of non-MHC-restricted lysis. Finally, we show that p46 and NKp44 are coupled to the intracytoplasmic transduction machinery via the association with CD3zeta or KARAP/DAP12, respectively; these associated molecules are tyrosine phosphorylated upon NK cell stimulation.
Figures

 ), anti-p46 (
), anti-p46 ( ), anti-CD56 (
), anti-CD56 ( ), anti-CD69 (
), anti-CD69 ( ), or anti-CD40L (
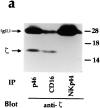
), or anti-CD40L ( ) mAbs at a 5:1 E/T ratio. All these mAbs are IgG1. Clones MA4 and MA5 were CD40L−, whereas clones MX340 and MX310 were CD40L+. (b) Biochemical characteristics. A 125I-surface labeled polyclonal NK population, derived from donor LM, was immunoprecipitated with Z231 (lanes A and E), anti-CD69 (lanes B and F), anti-CD16 (lanes C and G) or BAB281 (lanes D and H) mAbs. Samples were analyzed in an 11% SDS-PAGE under nonreducing (lanes A–D) or reducing (lanes E–H) conditions. (c) N-glycosidase F digestion. NKp44 (lanes A and B), CD69 (lanes C and D), CD16 (lanes E and F), or p46 (lanes G and H) molecules isolated from the surface-labeled NK cell population shown in b were treated or not with N-glycosidase F. Samples were run in an 11% SDS-PAGE under reducing conditions.
) mAbs at a 5:1 E/T ratio. All these mAbs are IgG1. Clones MA4 and MA5 were CD40L−, whereas clones MX340 and MX310 were CD40L+. (b) Biochemical characteristics. A 125I-surface labeled polyclonal NK population, derived from donor LM, was immunoprecipitated with Z231 (lanes A and E), anti-CD69 (lanes B and F), anti-CD16 (lanes C and G) or BAB281 (lanes D and H) mAbs. Samples were analyzed in an 11% SDS-PAGE under nonreducing (lanes A–D) or reducing (lanes E–H) conditions. (c) N-glycosidase F digestion. NKp44 (lanes A and B), CD69 (lanes C and D), CD16 (lanes E and F), or p46 (lanes G and H) molecules isolated from the surface-labeled NK cell population shown in b were treated or not with N-glycosidase F. Samples were run in an 11% SDS-PAGE under reducing conditions.
 ), anti-p46 (
), anti-p46 ( ), anti-CD56 (
), anti-CD56 ( ), anti-CD69 (
), anti-CD69 ( ), or anti-CD40L (
), or anti-CD40L ( ) mAbs at a 5:1 E/T ratio. All these mAbs are IgG1. Clones MA4 and MA5 were CD40L−, whereas clones MX340 and MX310 were CD40L+. (b) Biochemical characteristics. A 125I-surface labeled polyclonal NK population, derived from donor LM, was immunoprecipitated with Z231 (lanes A and E), anti-CD69 (lanes B and F), anti-CD16 (lanes C and G) or BAB281 (lanes D and H) mAbs. Samples were analyzed in an 11% SDS-PAGE under nonreducing (lanes A–D) or reducing (lanes E–H) conditions. (c) N-glycosidase F digestion. NKp44 (lanes A and B), CD69 (lanes C and D), CD16 (lanes E and F), or p46 (lanes G and H) molecules isolated from the surface-labeled NK cell population shown in b were treated or not with N-glycosidase F. Samples were run in an 11% SDS-PAGE under reducing conditions.
) mAbs at a 5:1 E/T ratio. All these mAbs are IgG1. Clones MA4 and MA5 were CD40L−, whereas clones MX340 and MX310 were CD40L+. (b) Biochemical characteristics. A 125I-surface labeled polyclonal NK population, derived from donor LM, was immunoprecipitated with Z231 (lanes A and E), anti-CD69 (lanes B and F), anti-CD16 (lanes C and G) or BAB281 (lanes D and H) mAbs. Samples were analyzed in an 11% SDS-PAGE under nonreducing (lanes A–D) or reducing (lanes E–H) conditions. (c) N-glycosidase F digestion. NKp44 (lanes A and B), CD69 (lanes C and D), CD16 (lanes E and F), or p46 (lanes G and H) molecules isolated from the surface-labeled NK cell population shown in b were treated or not with N-glycosidase F. Samples were run in an 11% SDS-PAGE under reducing conditions.
 ), anti-p46 (
), anti-p46 ( ), anti-CD56 (
), anti-CD56 ( ), anti-CD69 (
), anti-CD69 ( ), or anti-CD40L (
), or anti-CD40L ( ) mAbs at a 5:1 E/T ratio. All these mAbs are IgG1. Clones MA4 and MA5 were CD40L−, whereas clones MX340 and MX310 were CD40L+. (b) Biochemical characteristics. A 125I-surface labeled polyclonal NK population, derived from donor LM, was immunoprecipitated with Z231 (lanes A and E), anti-CD69 (lanes B and F), anti-CD16 (lanes C and G) or BAB281 (lanes D and H) mAbs. Samples were analyzed in an 11% SDS-PAGE under nonreducing (lanes A–D) or reducing (lanes E–H) conditions. (c) N-glycosidase F digestion. NKp44 (lanes A and B), CD69 (lanes C and D), CD16 (lanes E and F), or p46 (lanes G and H) molecules isolated from the surface-labeled NK cell population shown in b were treated or not with N-glycosidase F. Samples were run in an 11% SDS-PAGE under reducing conditions.
) mAbs at a 5:1 E/T ratio. All these mAbs are IgG1. Clones MA4 and MA5 were CD40L−, whereas clones MX340 and MX310 were CD40L+. (b) Biochemical characteristics. A 125I-surface labeled polyclonal NK population, derived from donor LM, was immunoprecipitated with Z231 (lanes A and E), anti-CD69 (lanes B and F), anti-CD16 (lanes C and G) or BAB281 (lanes D and H) mAbs. Samples were analyzed in an 11% SDS-PAGE under nonreducing (lanes A–D) or reducing (lanes E–H) conditions. (c) N-glycosidase F digestion. NKp44 (lanes A and B), CD69 (lanes C and D), CD16 (lanes E and F), or p46 (lanes G and H) molecules isolated from the surface-labeled NK cell population shown in b were treated or not with N-glycosidase F. Samples were run in an 11% SDS-PAGE under reducing conditions.




References
-
- Moretta A, Bottino C, Vitale M, Pende D, Biassoni R, Mingari MC, Moretta L. Receptors for HLA-class I molecules in human natural killer cells. Annu Rev Immunol. 1996;14:619–648. - PubMed
-
- Moretta A, Biassoni R, Bottino C, Pende D, Vitale M, Poggi A, Mingari MC, Moretta L. Major histocompatibility complex class I–specific receptors on human natural killer and T lymphocytes. Immunol Rev. 1997;155:105–117. - PubMed
-
- Lanier LL. NK cells: from no receptors to too many. Immunity. 1997;6:371–378. - PubMed
-
- Valiante NM, Lienert K, Shilling HG, Smits BJ, Parham P. Killer cell receptors: keeping pace with MHC class I evolution. Immunol Rev. 1997;155:155–164. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

